Peerless Process Of Exposing Iron Nails In Wet Cloth

Observe your nails 24 hours later.
Process of exposing iron nails in wet cloth. If you dont have a hairdryer steam off the letters using an iron and a wet cloth. In this practical students put iron nails in various conditions including wet dry air-free and salty to find out what causes iron to rust. Answer Stains caused by rusted nails will bleed through the topcoat of even the most durable paints unless a stain-resistant solvent-based or acrylic latex primer has been applied first.
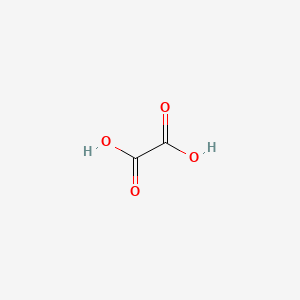
When substances made of iron are exposed to oxygen and moisture water rusting takes place. A metal object corrodes when it is exposed to the open air and bad weather. The chemical composition of rust is typically hydrated ironIII oxide Fe2O3nH2O and under wet conditions may include ironIII oxide-hydroxide FeOOH.
The nail plate itself is the hard substance on the back of the finger or toe. It could be left set up for longer if necessary. Corrosion is a natural process which converts a refined metal to a more chemically-stable form such as its oxide hydroxide or sulfide.
Rust is a general term for iron oxides formed by the reaction of iron with oxygen. IronIII oxide or ferric oxide where the iron atom exhibits an oxidation state of 3. If you are painting new exterior construction where non-galvanized nails have been used its advisable to spot-prime the nailheads and any knots in the wood prior to applying the topcoat.
The World Health Organization lists iron deficiency as the most common nutritional disorder in the world. Investigating the rusting oxidation of iron Materials. If the metal is iron we call this change rusting and the weaker flaky brown compound that is formed is rust.
Nails are exposed to rain heat and other factors that can increase oxidation potential. Put your iron on the highest setting and place it on top of the cloth. Your body uses iron for a variety of functions crucially to help transport oxygen from the lungs to your organs and tissues.