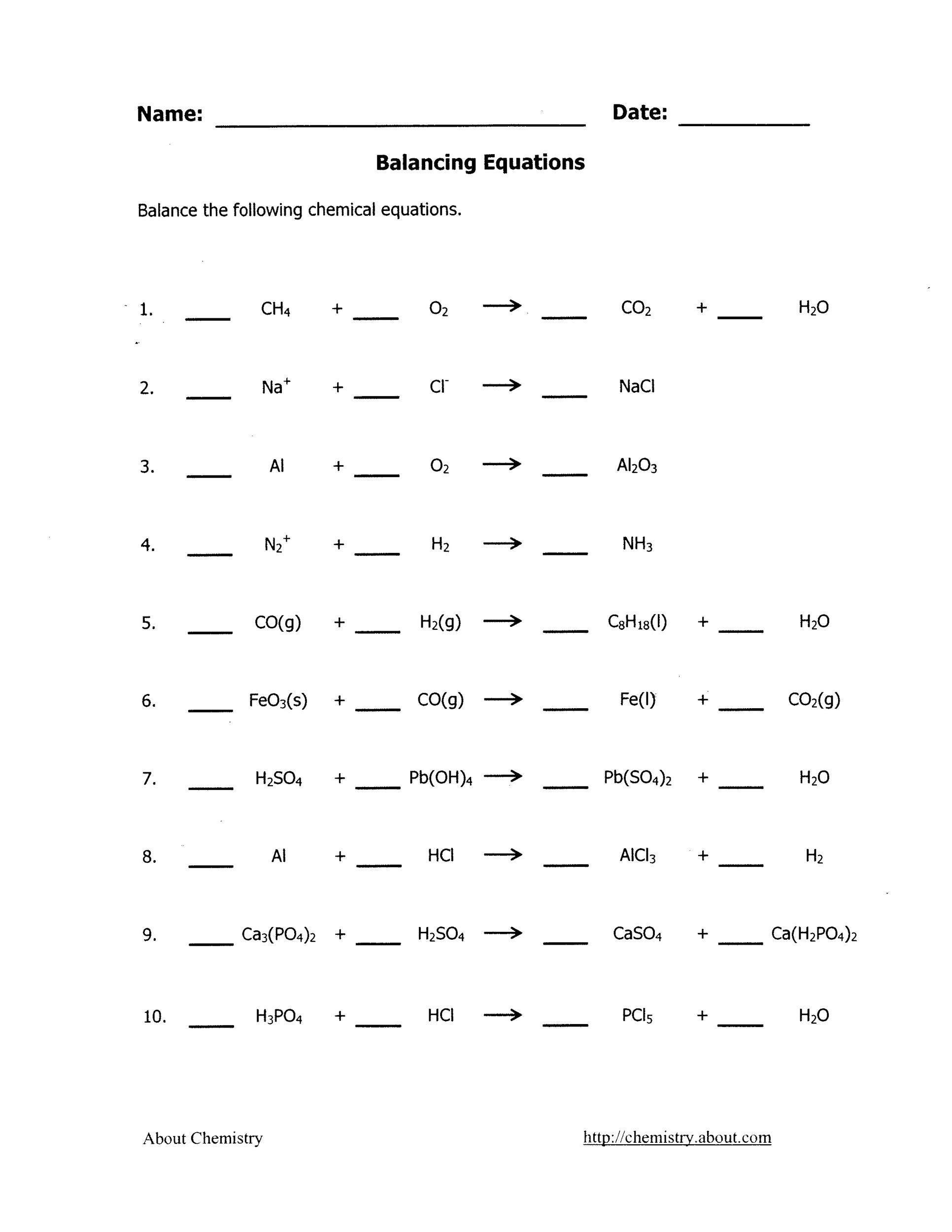
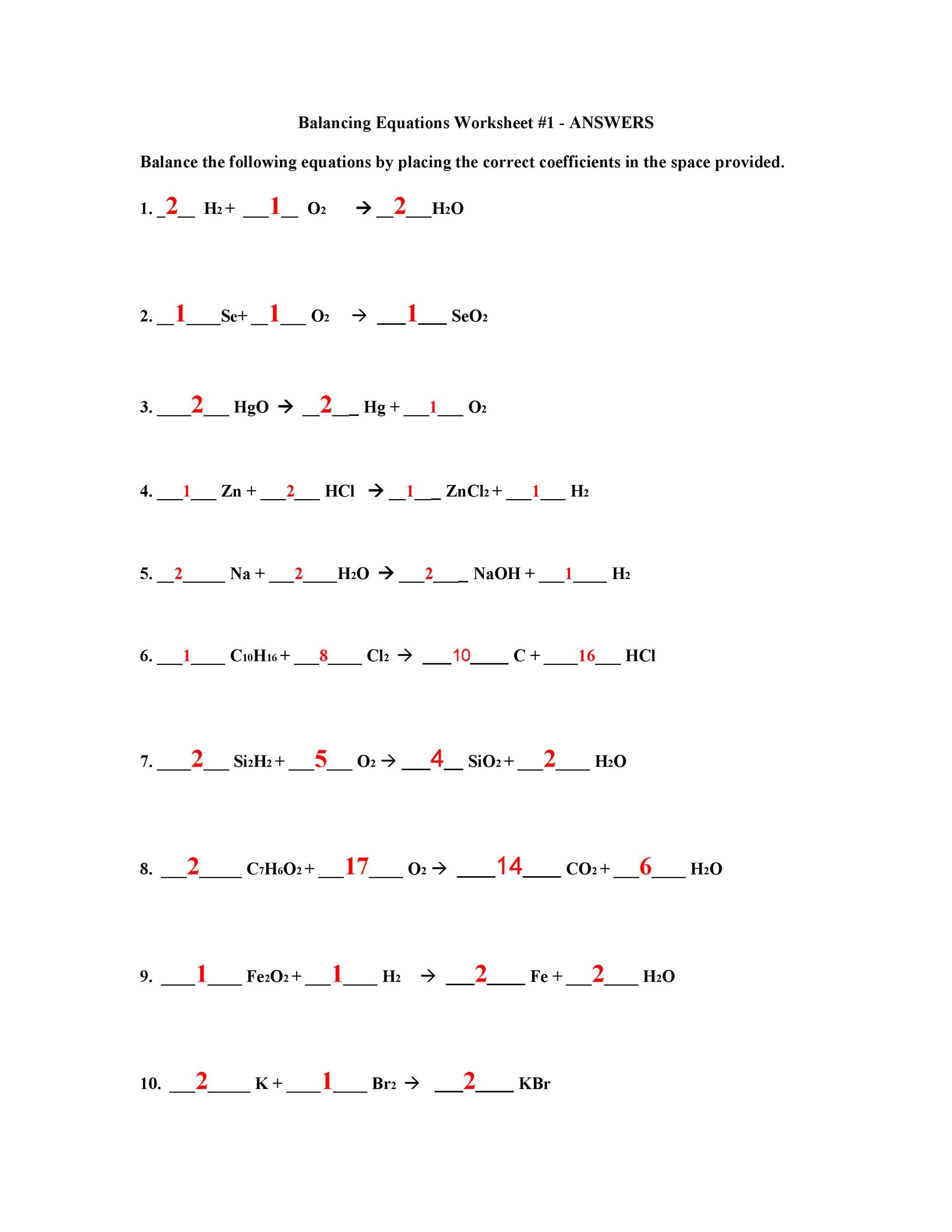
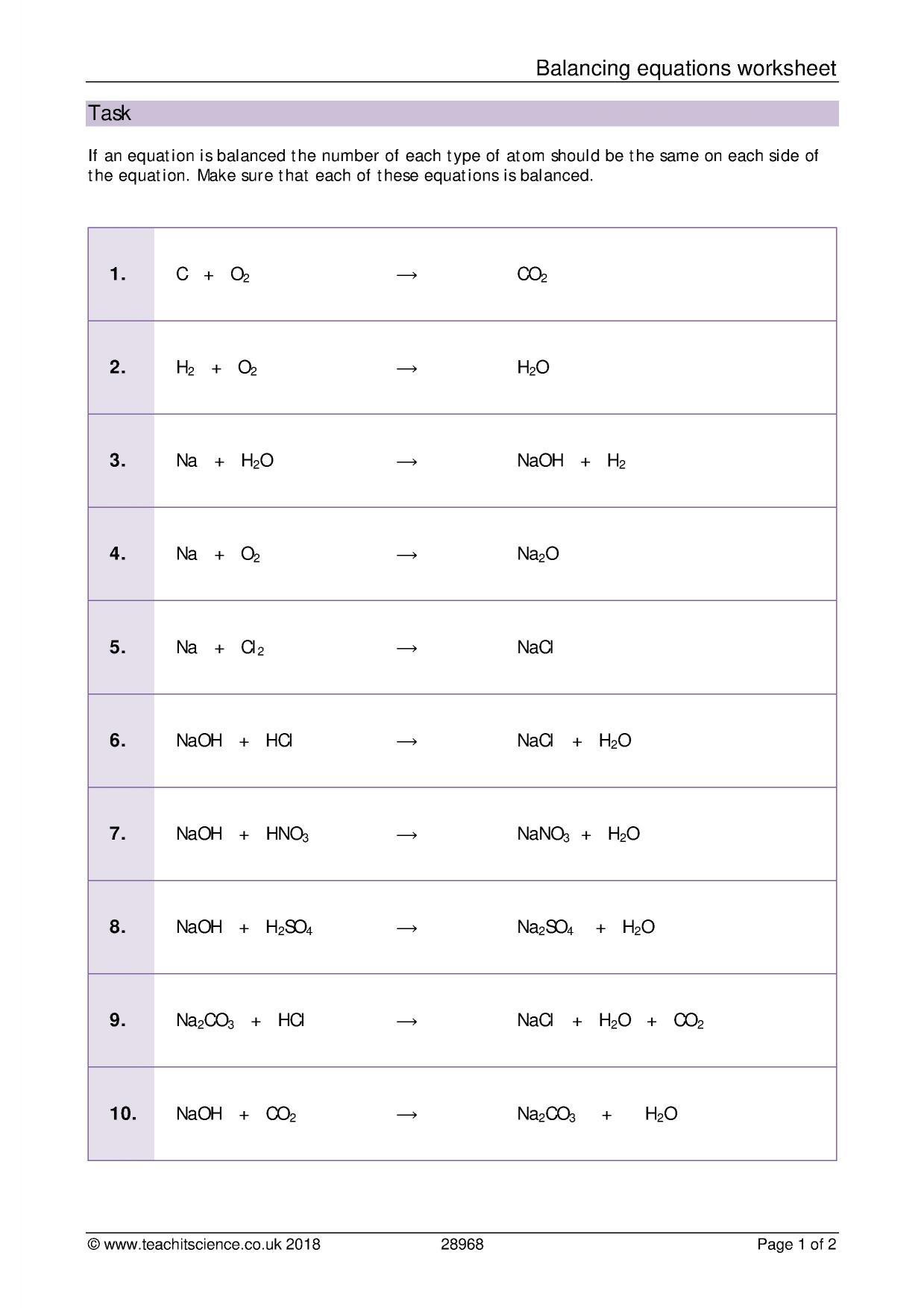
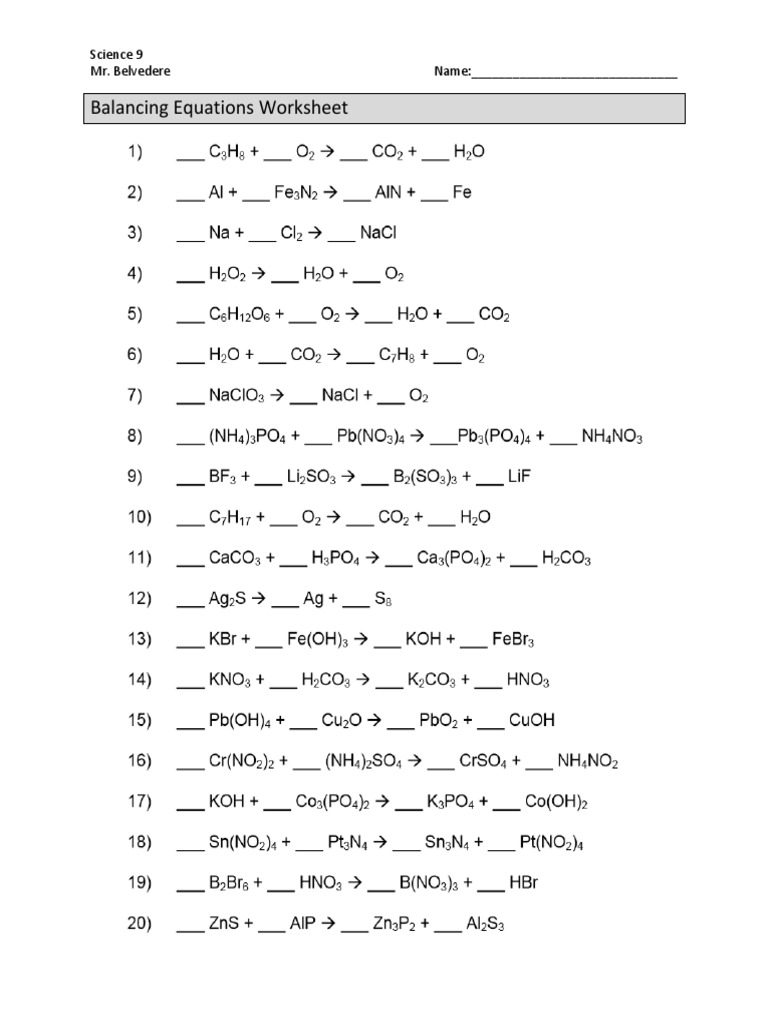
Favorite Balancing Equation Exercise
By Anne Marie Helmenstine PhDSep 11 20162 mins to read.
Balancing equation exercise. Ol NaOHaq H. For the things we have to. SiO 2 2C Si 2CO 31.
Begin by counting each kind of atom on both sides of the arrow. Balance the following equations of redox reactions. Question 1 Balance the following equation.
The Na and O atoms are balancedone Na and one O on each sidebut there are two H atoms on the left and three H atoms on the right. 2NaHCO 3 Na 2 CO 3 CO 2 H 2 O 32. On the left side of the arrow you will find the reactant side.
We have three elements. Add the two half-reactions together and cancel out common terms. Separate the redox reaction into two half reactions.
If you are new to balancing chemical equations this video will give you the practice you need to be successful. Write and balance the chemical equation described by Exercise 4. Student exploration balancing chemical equations search results.
2Mg Cl 2 MgCl 2 27. Assign oxidation numbers to all elements in the reaction. Fe Au Co Br C O N F.