Smart Baking Soda Formation Equation

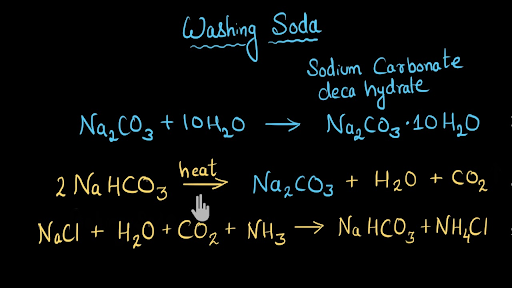
2 NaHCO3 s Na2CO3 s CO2 g H2O g Like most chemical reactions the rate of the reaction depends on temperature.
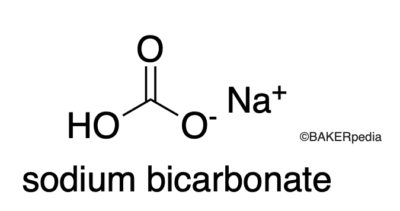
Baking soda formation equation. Sodium bicarbonate commonly known as baking soda is a chemical compound with the formula NaHCO 3. The formula of baking soda is NaHCO3. Baking soda is sodium bicarbonate and both sodium and bicarbonate can be poisonous if swallowed in large amounts.
The Medieval Egyptians first quarried Natron a natural. It has a slightly salty flavor. Baking soda or baking powder has various chemical names like sodium bicarbonate Na 2 CO 3 bicarbonate of soda sodium hydrogen carbonate NaHCO 3 etc.
Its main chemical formula is NaHCO 3 and its prepared industrially by Solvay process. It is also called sodium bicarb or simply bicarb. The compound is a salt that dissociates into sodium Na cation and carbonate CO 3- anions in water.
2 NaHCO3 s Na2CO3 s CO2 g H20 1 AH -1293 kJ Table 1. Write the thermochemical equation for the standard heat of formation of baking soda using the chemical reaction and data given. Baking Soda is also defined as Sodium Bicarbonate.
The use of baking soda is especially popular in pancakes and waffles since the high cooking temperatures of 350. It is a salt composed of a sodium cation and a bicarbonate anion. Specifically the baking soda a base reacts with the acid to give you carbon dioxide gas water and salt.
The balanced equation for the decomposition of sodium bicarbonate into sodium carbonate carbon dioxide and water is. What is baking powder. Baking soda is used to prepare cakes in order to ensure that cakes rise as they bake.