Matchless Reaction Of Khp With Naoh
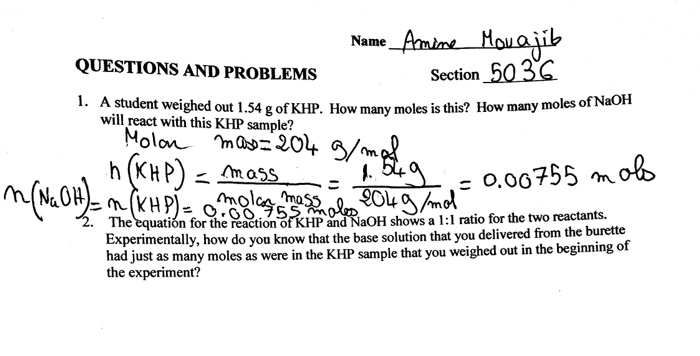
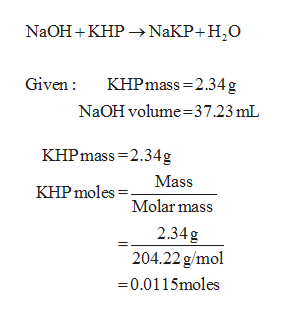
The molar mass of KHP is approximately 20422 gmol.
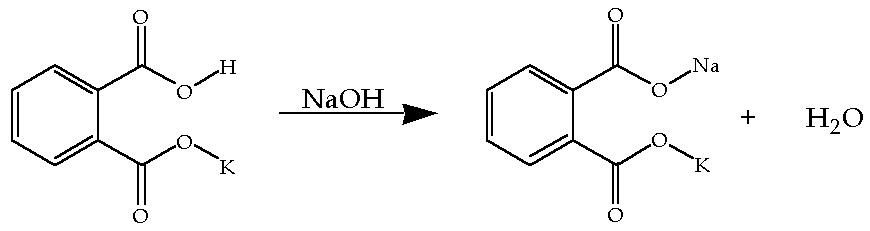
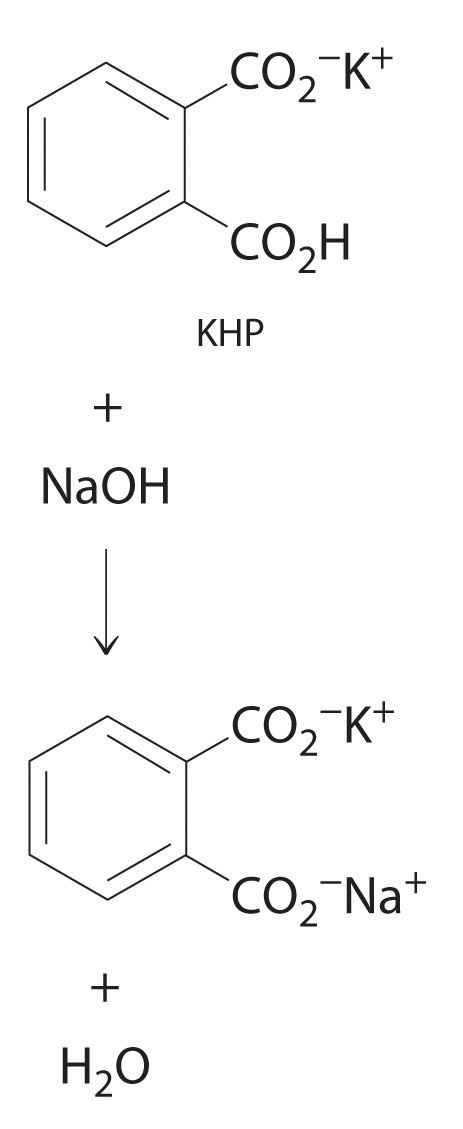
Reaction of khp with naoh. The equation is solved for MNaOH. Number of moles of KHP neutralized number of moles of NaOH added Restated in an equation which holds at the equivalence point. 154g of KHP is equivalent to 000754 mol of KHP.
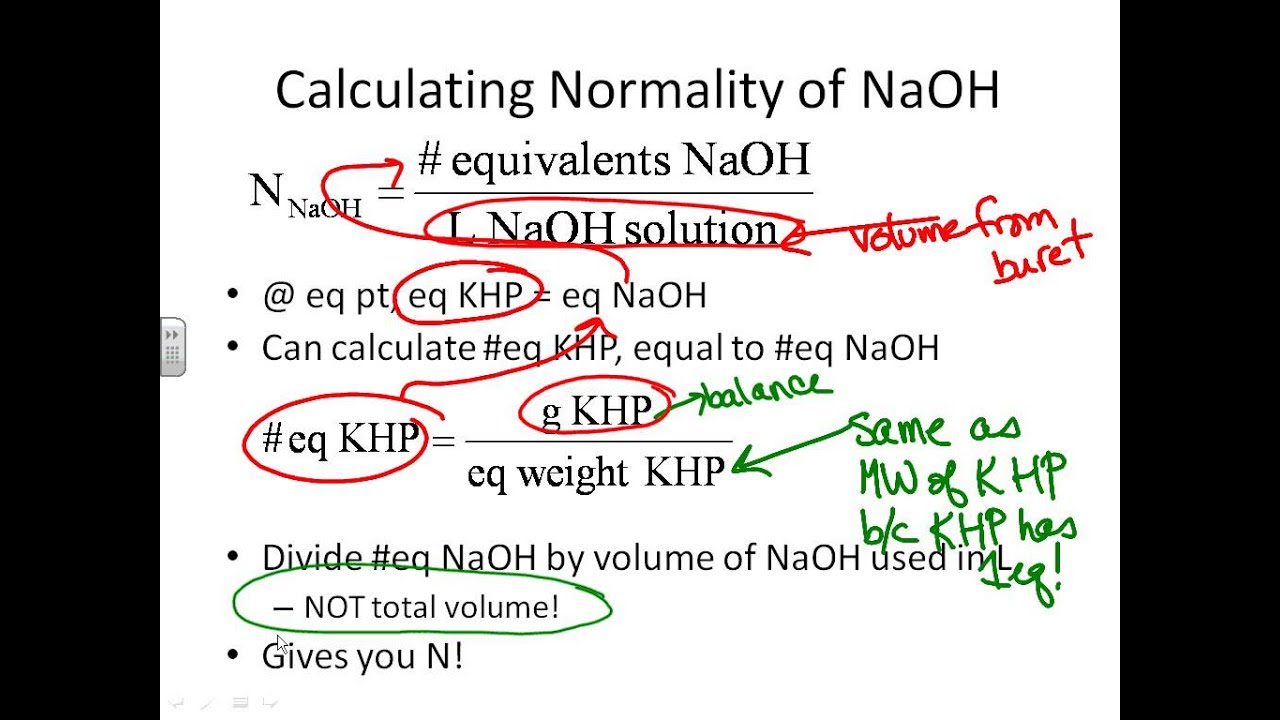
The volume of NaOHaq required to reach the equivalence point would be larger than if the contaminant were not present The volume of NaOHaq required to reach. Standardization of a H 2 SO 4 Solution using KHP In a second procedure a 2500 mL sample of H 2 SO 4 was titrated with the standardized NaOH solution from Example 1. The products are water and a salt.
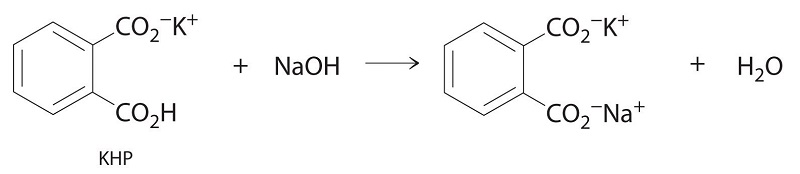
In this reaction as well one mole of KHP completely reacts with one mole of NaOH. Write the balanced chemical equation for the reaction of KHP with NAOH. Once the KHP completely turns into KNaC8H4O4 during the titration you know that the solution reached the equivalence point and the reaction is a weak base.
1 mole of NaOH reacts per mole of KHP so00754 mol of NaOH are needed. Were reacting KHP with NaOH. The titration of NaOH with KHP involves adding NaOH from the burette to a known volume of KHP.
You weigh out 06101 grams of KHP and dissolve this material in water. My question is where does the KHP come into this equation. Slowly add the NaOH solution to the flask containing the KHP swirling gently during the addition Continue adding the NaOH until the endpoint is reached which is the first sign of a pink color that remains after at least 30 seconds of swirling.
How much NaOH does it take to completely react with a sample of KHP. Making 1 N solution of NaOH To make 1 N solution dissolve 4000 g of sodium hydroxide in water to make volume 1 liter. They both are reacting with each other.