Impressive Acid Rain Chemical Formula
Acids in acid rain promote the dissolution of calcium carbonate by reacting with the carbonate anion.
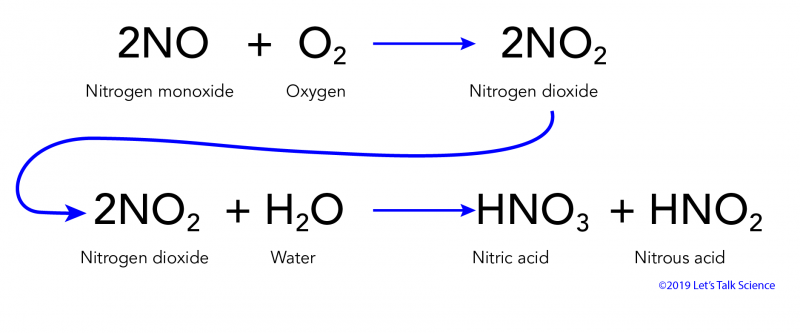
Acid rain chemical formula. 2NO 2 HOH - HNO 2 HNO 3. Acid rain is a popular expression for the more scientific term acid deposition which refers to the many ways in which acidity can move from the atmosphere to Earths surface. Forms of Acid Rain.
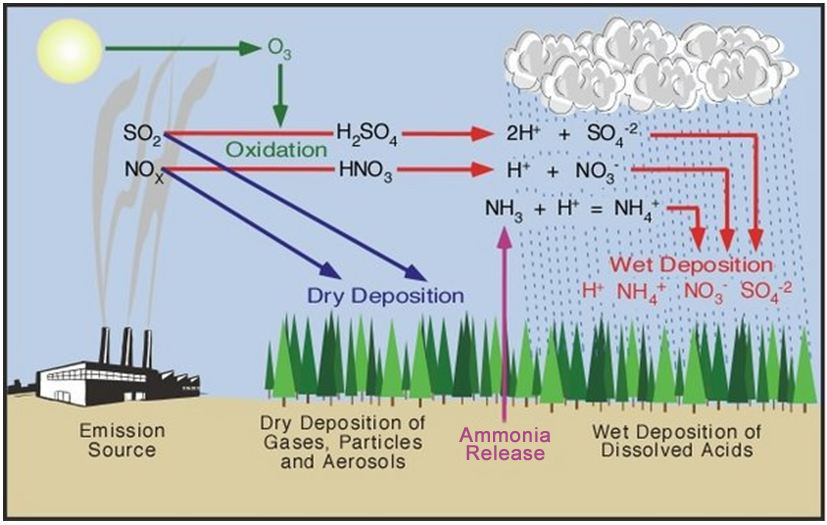
16121 C O 2 g H 2 O l H 2 C O 3 a q H a q H C O 3 a q. The nitrogen oxides and sulfur oxides that form acid rain come from both man-made and natural sources. Acid rain is caused by a chemical reaction that begins when compounds such as sulphur dioxide and oxides of nitrogen are released into the air.
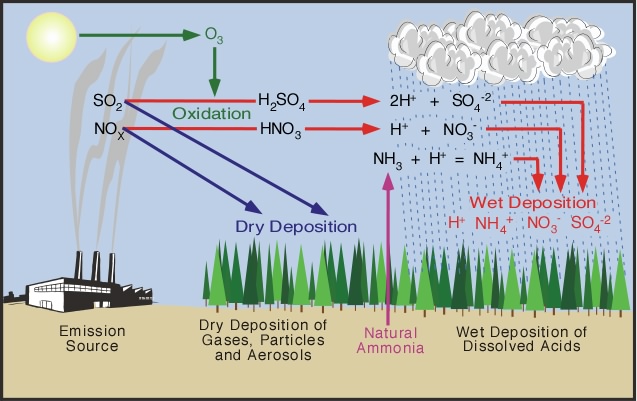
Acid rain refers to any kind of precipitation that transports nitrogen and sulfur compounds to the Earths surface. They may also precipitate in other forms such as sleet snow hail or even fog. They get mixed with the rain and fall on the earths surface as acid rain.
Limestone is one familiar form of calcium carbonate. The precursors or chemical forerunners of acid rain formation result from both natural sources such as volcanoes and decaying vegetation and man-made sources primarily emissions of sulfur dioxide SO 2 and nitrogen oxides NO x resulting from fossil. Acidic rain is a complex mixture of nitrous nitric sulfurous and sulfuric acids which all combine to lower the pH.
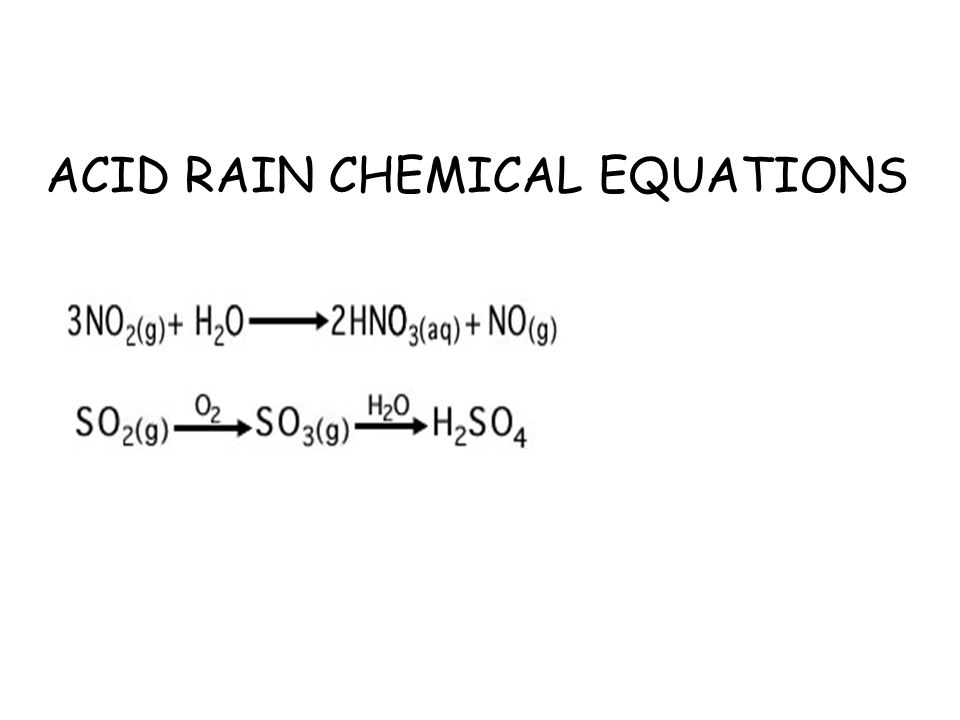
This is due to the presence of sulphurous acid sulphuric acid and nitric acid in rain water. The reaction equation for acid rain produced from nitrogen oxides is. This produces a solution of bicarbonate.
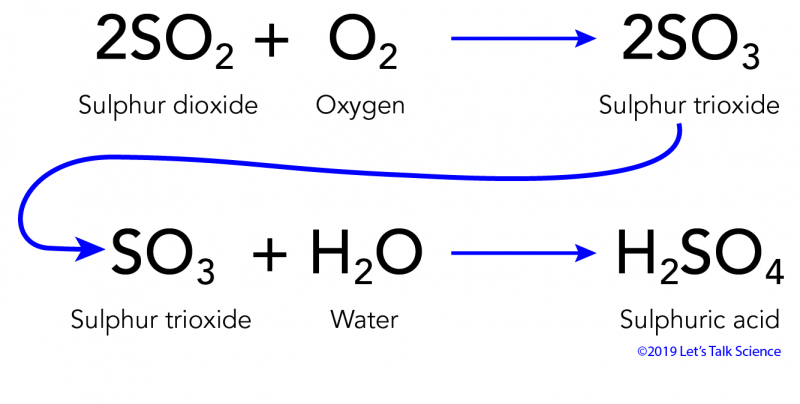
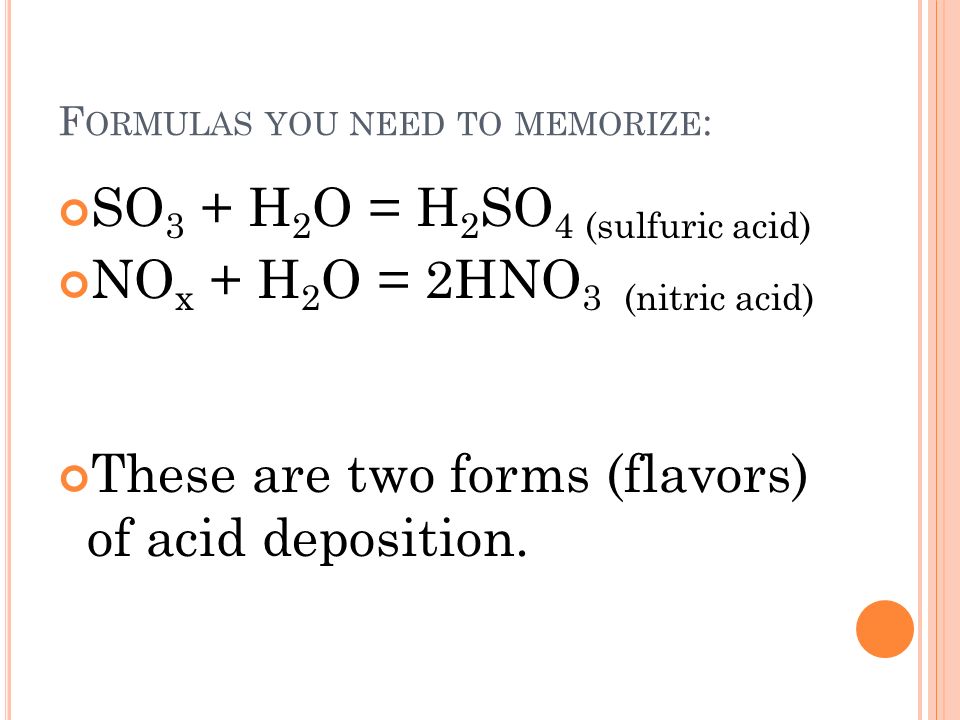
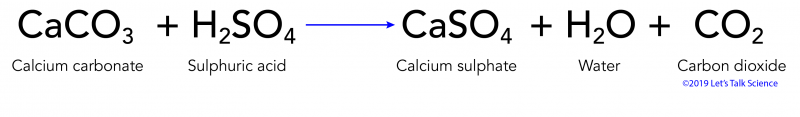
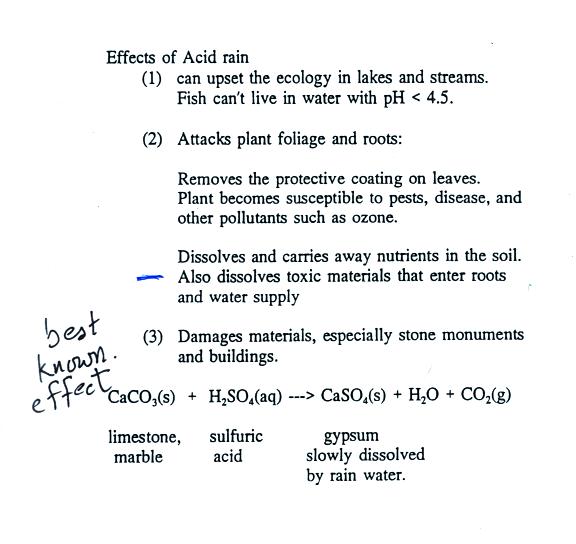
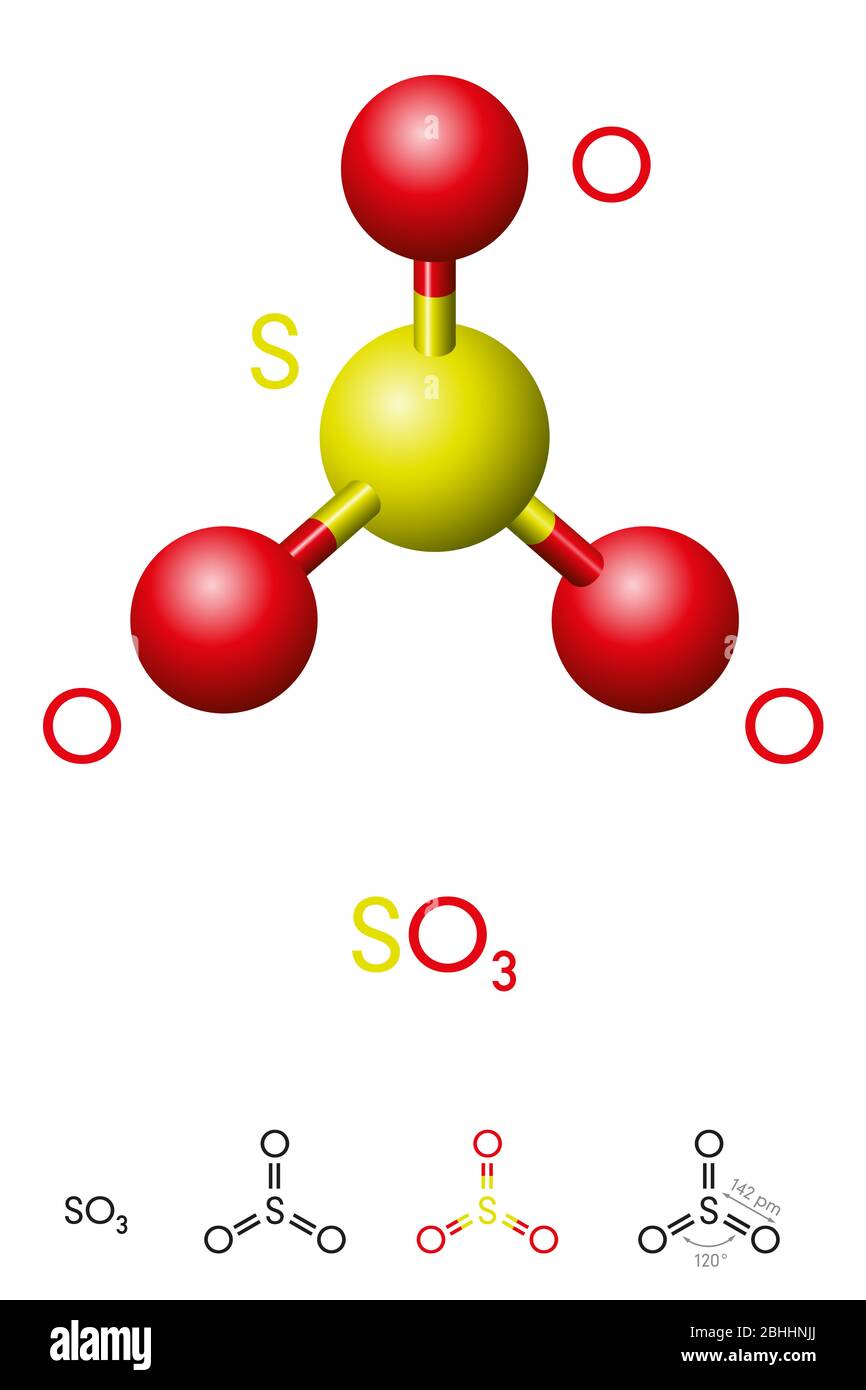
Calcium carbonate sulphuric acid calcium sulfate water carbon dioxide Chemical reaction of calcium carbonate with sulphuric acid to form calcium sulfate water and. 2SO 2 g O 2 g 2H 2 O I 2H 2 SO 4 aq Acid rain occurs when the pH of rain water falls between 20 to 55. Sulfuric acid H 2SO4 nitric acid H N O3 and carbonic acid H 2CO3 are the major components of acid rain.