Beautiful Work Acid Rain Chemical Equation
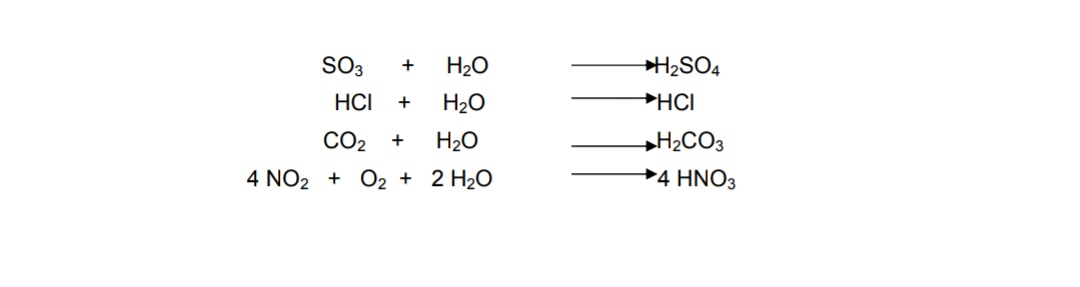
Acid rain pH 41 1982.
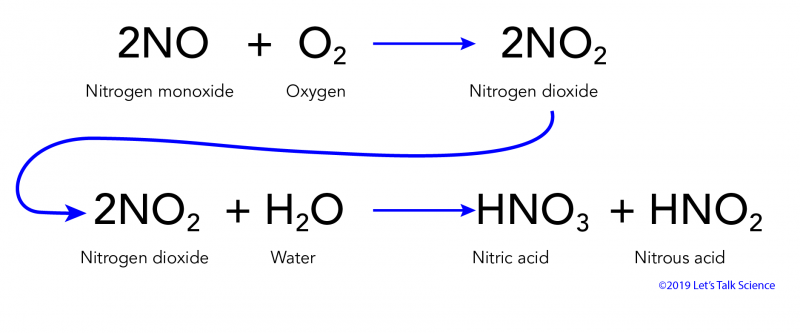
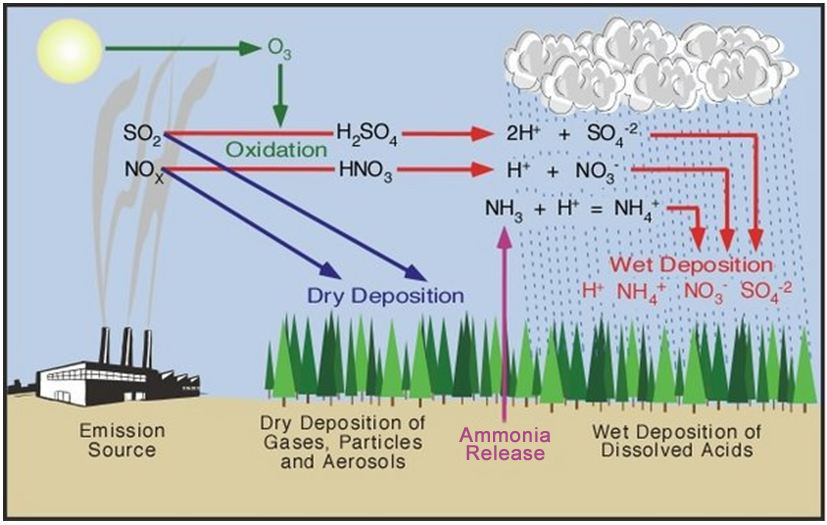
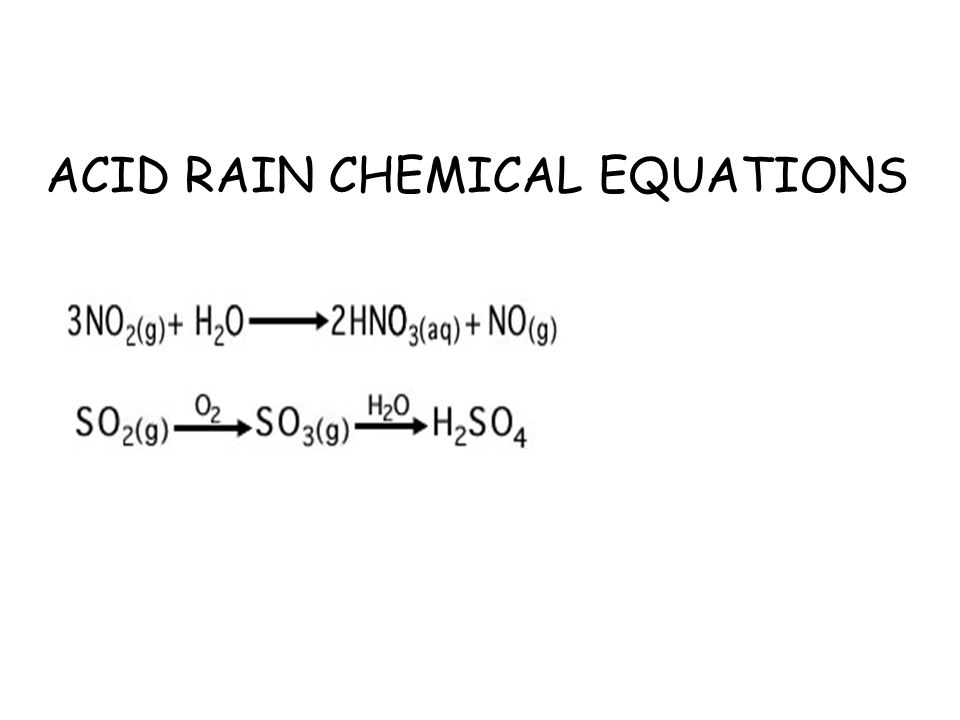
Acid rain chemical equation. Nitric acid is a component of acid rain that forms when gaseous nitrogen dioxide pollutant reacts with gaseous oxygen and liquid water to form aqueous nitric acid. Acid rain pollutes the air and corrodes buildings monuments and statues made of metals and marble. Carbon dioxide in atmosphere reacts with water.
Metals like iron and calcium carbonate react with the acid in the rain slowly as follows. The nitrogen oxides and sulfur oxides that form acid rain come from both man-made and natural sources. Acid rain - acid rain - Chemistry of acid deposition.
Acid rain or acid deposition is a broad term that includes any form of precipitation with acidic components such as sulfuric or nitric acid that fall to the ground from the atmosphere in wet or dry forms. 12 kg C 32 kg O 34 kg CO2 Carbonic acid H2CO3 formation. This can include rain snow fog hail.
Fog pH 18. This is a simplified representation of this reaction. CO2 reacts with water to form carbonic acid Equation 1.
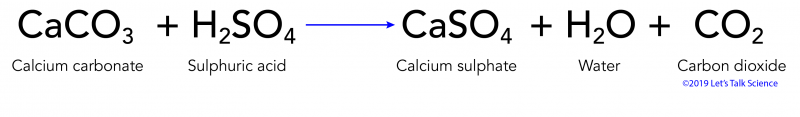
Fe s H 2 SO 4 aq FeSO 4 aq H 2 g CaCO 3 s H 2 SO 4 aq CaSO 4 s CO 2 g H 2 O l. Effect of Limestone Calcium carbonate CaCO 3 is a very common mineral. Carbon dioxide reacts with water to form carbonic acid Equation 1.
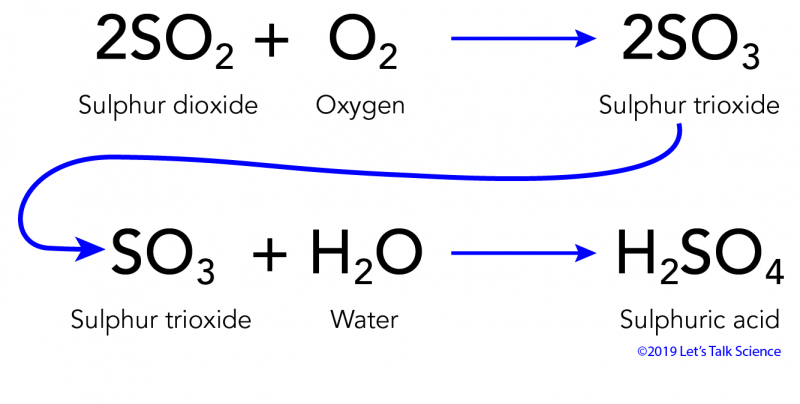
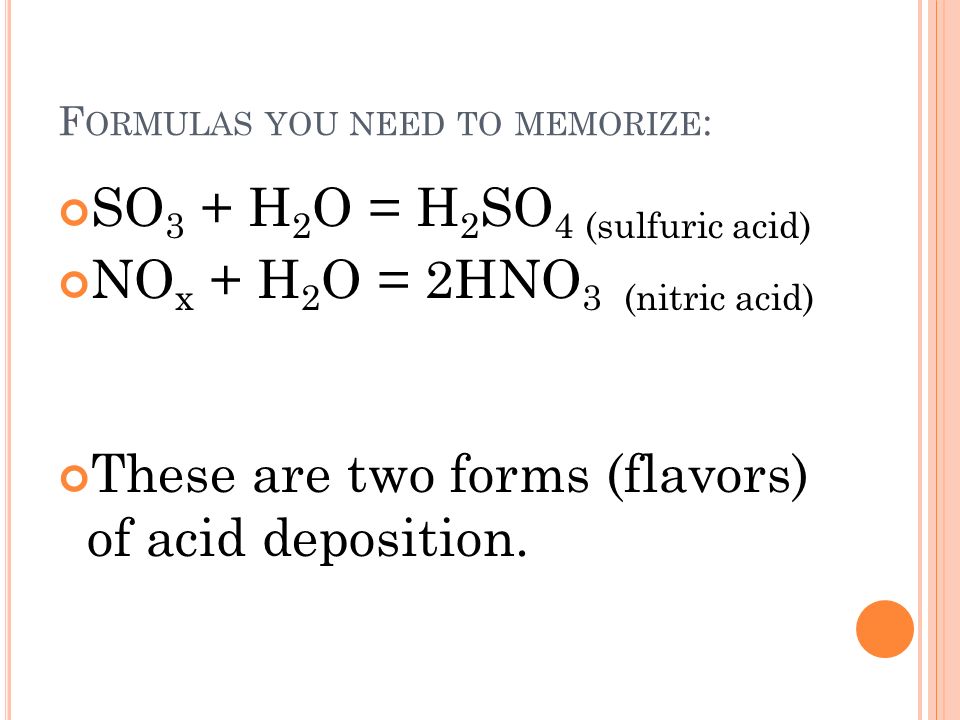
Calcium carbonate sulphuric acid calcium sulfate water carbon dioxide Chemical reaction of calcium carbonate with sulphuric acid to form calcium sulfate water and. Carbonic acid then dissociates to give the hydrogen ion H and the hydrogen carbonate ion HCO3- Equation 2. Sulfur dioxide water - sulfurous acid sulfur trioxide water - sulfuric acid nitrogen dioxide water - nitric acid nitrous acid.