Casual Propane + Oxygen Balanced Equation

CaOH 2 H 3 PO 4.
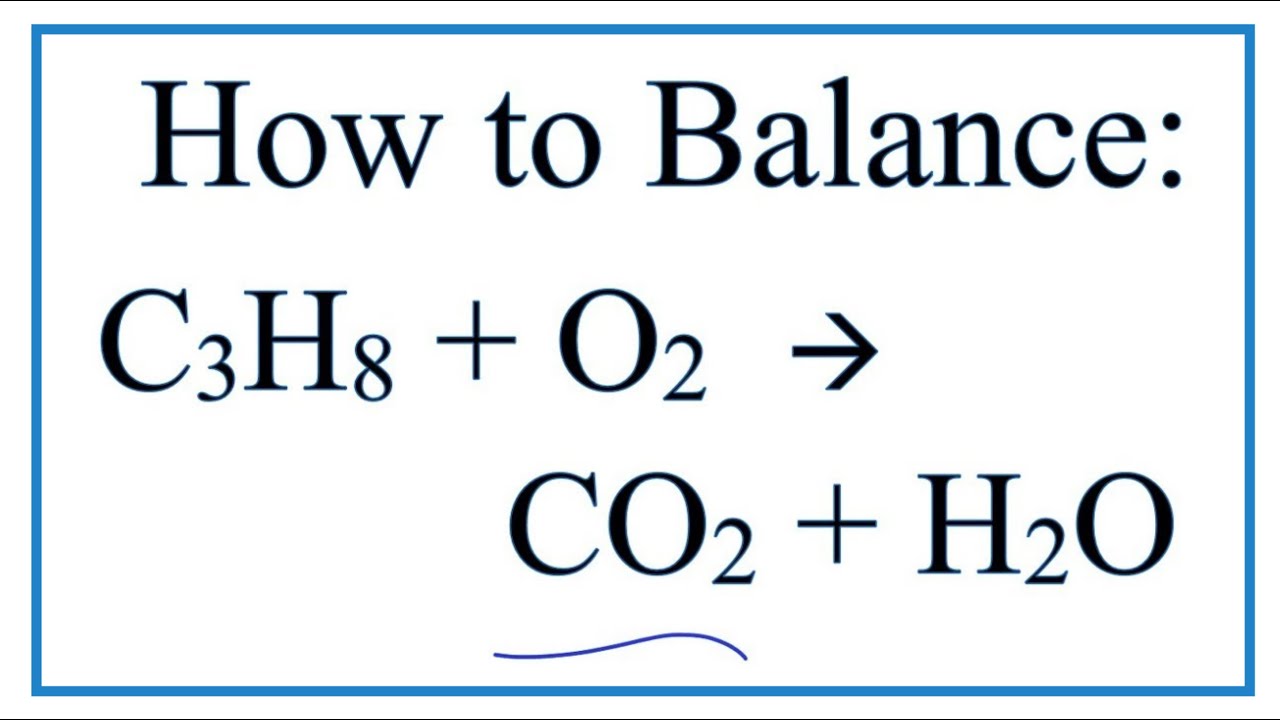
Propane + oxygen balanced equation. The above method is more of theoretical interest than of practical utility. - - The principle of all bomb calorimetry is tiiat a known weight of sample is ignited-electrically and burned in an excess of oxygen in the bomb. What type of reaction is propane and oxygen.
When Propane C3H8 Reacts With Oxygen Carbon Dioxide And Water Are Produced. The propane equation for complete combustion of propane involves propane and oxygen as fuel input and carbon dioxide water heat and possible carbon monoxide as the outputs. Non-toxic three-carbon alkane gas.
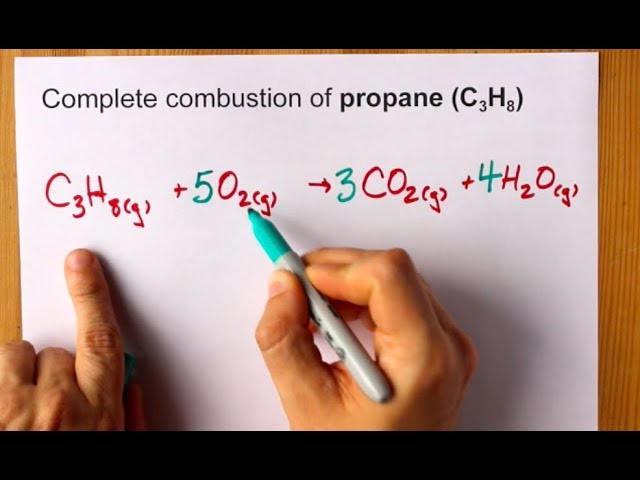
B Calculate the number of liters of carbon dioxide measured at STP that could be produced from 745 g of propane. Write a balanced chemical equation for the combustion of gaseous propane in gaseous oxygen to produce gaseous carbon dioxide and liquid water. The balanced chemical equation for the combustion of propane is.
What happens when ethane reacts with oxygen. C 3 H 8 5 O 2 3 CO 2 4 H 2 O heat. However in the equation 2H2 O2 --2H2O there are four hydrogen atoms and two oxygen atoms on the left and four hydrogen atoms and two oxygen.
Oxygen to form gaseous carbon dioxide and gaseous water. What type of reaction is propane and oxygen. Propane oxygen carbon dioxide water heat.
What type of reaction is C3H8 O2 CO2 H2O. Since the product side contains 32 41 10 moles of oxygen reactant O2 must be multiplied by 5 to have 10 moles as well. The balanced chemical equation.