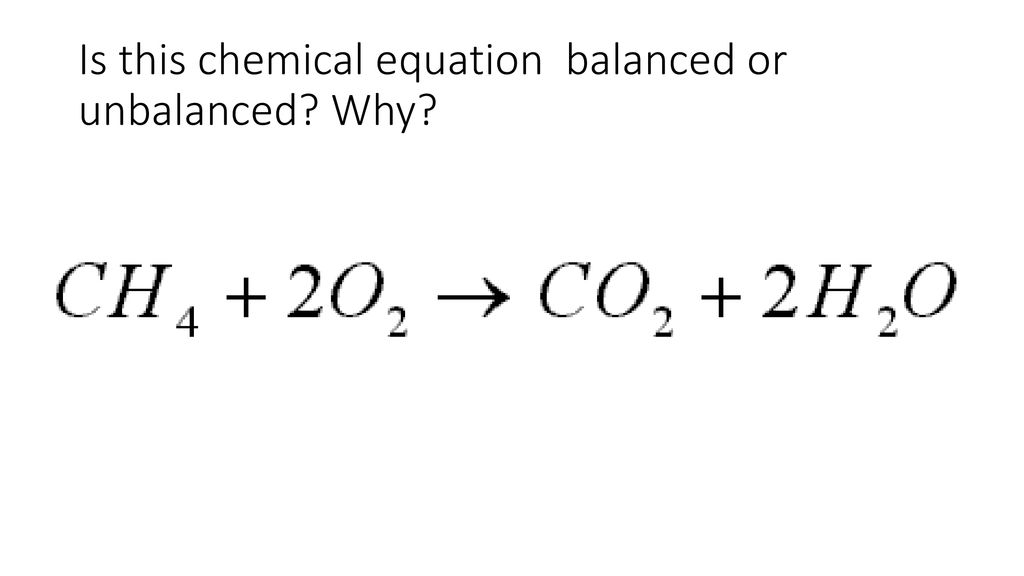Perfect Unbalanced Equation Definition

It does not tell.
Unbalanced equation definition. For example this equation for the reaction between iron oxide and carbon to form iron and carbon dioxide is unbalanced with respect to mass. In order for the two sides to be balanced we need to put a little more mass on the left side until they are the same mass. Here again phase angle information is lost since only magnitudes are considered.
Ionic charges are not yet supported and will be ignored. Lets take a look at this scale. An unbalanced allotment of resources.
We can see that it is unbalanced with the right red side weighing more than the left blue side. Unbalanced Force Equation Suppose F1 and F2 are two unequal forces acting on an object in opposite directions. Demerits of Skeletal or Unbalanced Chemical equation.
The chemical equation in which the total number of atoms of each element in reactants and products are not equal. F1 F2 0. In this example the reactants are glucose C 6 H 12 O 6 and oxygen O 2 and the products are carbon dioxide CO 2 and water H 2 O The unbalanced chemical equation is C6H12O6 O2 CO2 H2O Step 2.
Then by definition of an unbalanced force the net force acting on the object is non-zero. The equation where number of atoms of each element are not same on both sides is known as unbalanced chemical equation. ə nst ɪˈkweɪʒ ə n us ˌbæl.
Add coefficients the numbers in front of the formulas so the number of atoms of each element is the same on both sides of the equation. The balanced equation will appear above. An unbalanced equation is typically seen on college campuses sporting events workout facilities as well as in the general population.