Beautiful Work Strontium Nitrate And Sulfuric Acid

Strontium nitrate can aid in eliminating and lessening skin irritations.
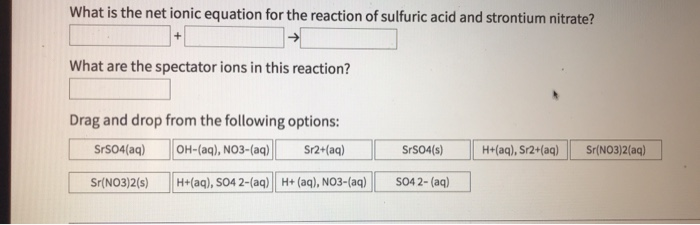
Strontium nitrate and sulfuric acid. When mixed with glycolic acid strontium nitrate reduces the sensation of skin irritation significantly better than using glycolic acid alone. SO 42 aq H aq HSO 4 aq L SrSO 4. My thoughts based on these results narrow the cation down to lithium and strontium.
Write the balanced formula unit equation the total ionic equation and the net ionic equation for this reaction. Stochiometric quantities of both reagents are recommended for maximum yield. Sr II is precipitated by sulfate ions at neutral or slightly acidic solutions.
Strontium chloride - solid. Adding sulfuric acid to Barium nitrate- insoluble and precipitate forms. On the basis of this information suggest what you would observe when dilute sulfuric acid is added to a solution of strontium nitrate with a concentration of 5 g per 100 g of water at 25 C.
Reactions with dilute hydrochloric acid. Aqueous solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. Sr 2 aq SO 42 aq SrSO 4 s In strong acidic solutions SrSO 4 dissolves as the sulfate concentration becomes to small.
Nitric acid reacts with strontium hydroxide producing soluble strontium nitrate and water. Yes a white ppt of BaSO4 forms. This is a typical acid base reaction.
Strontium sulfate has a lower solubility in water at 25 C than calcium sulfate. A yellow colored gas formed when 4 drops of sulfuric acid were added with substance in test tube. Although chromate ion does not give a precipitate in neutral or acid solutions of Sr 2 yellow SrCrO 4 precipitates from slightly basic solution.