Fun Rusting Definition Chemistry

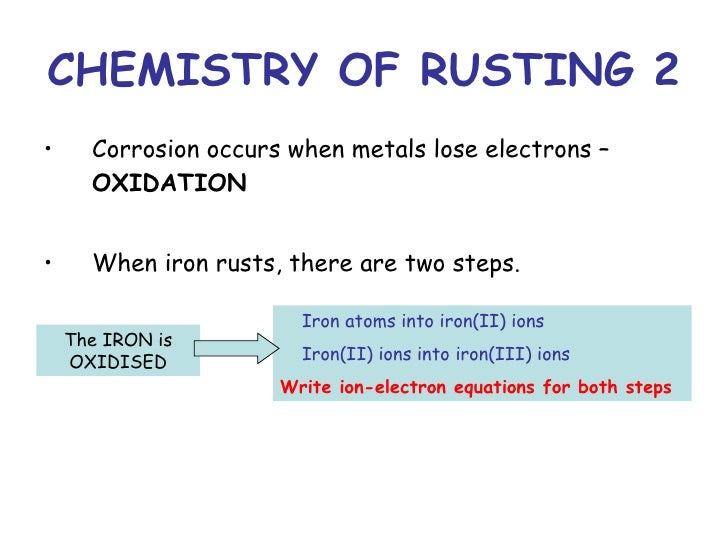
Rusting of iron is a chemical change because a new substance called iron oxide is formed during this process.
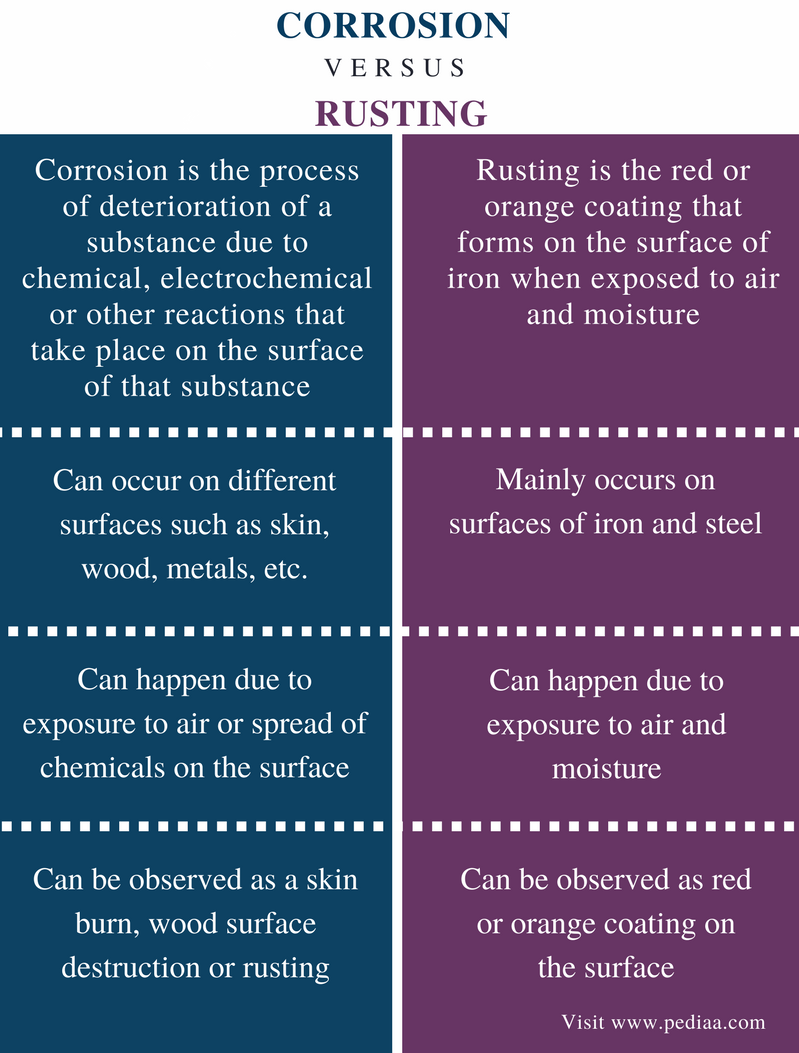
Rusting definition chemistry. Rusting is a chemical reaction between iron water and oxygen to form the compound iron III oxide. Oxygen and water must be present for rusting to occur. Rusting is a redox process and it occurs faster in salty water since the presence of sodium chloride increases the electrical conductivity of the water.
Rusting is a part of corrosion. Definition Conditions Prevention of Rusting of Iron. Any of various powdery or scaly reddish-brown or reddish-yellow hydrated ferric oxides and hydroxides formed on iron and iron-containing materials by low-temperature oxidation in the presence of water.
Iron water oxygen. Technically its iron oxide hydrate because pure iron oxide isnt rust. Rust is an iron oxide a usually reddish-brown oxide formed by the reaction of iron and oxygen in the catalytic presence of water or air moisture.
Rust forms when iron or its alloys are exposed to moist air. Corrosionpedia explains Chemical Corrosion. Related to Old Saxon Old High German rost.
Stay tuned with BYJUS to learn more about other concepts such as the rusting of iron. Iron water oxygen. A reddish brown deposit called rust forms over a piece of iron when it is exposed to moist air for some time.
Rust can also effect iron alloys such as steel. Was this answer helpful. To deteriorate or cause to deteriorate through some debilitating influence or lack of use.