Impressive Propane Burning Equation

Incomplete combustion produces carbon monoxide which is a poisonous gas.
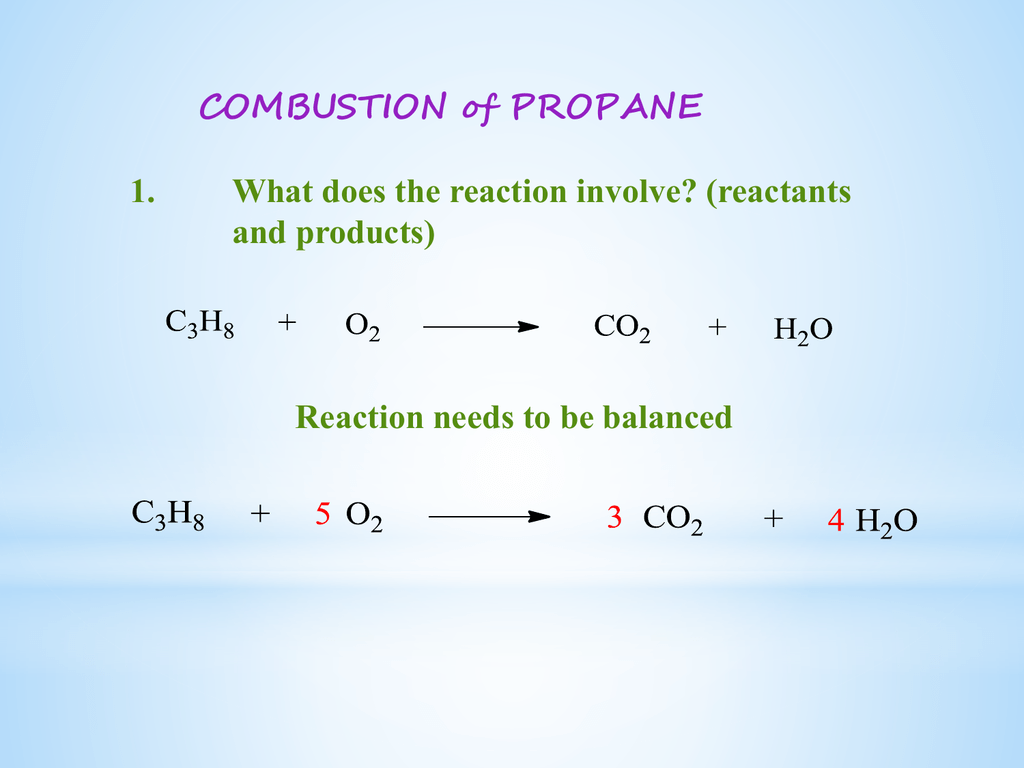
Propane burning equation. Explained Process of Combustion of Propane. PROPANE is an energy-rich gas that is related to petroleum and natural gas. The propane equation for complete combustion of propane involves propane and oxygen as fuel input and carbon dioxide water heat and possible carbon monoxide as the outputs.
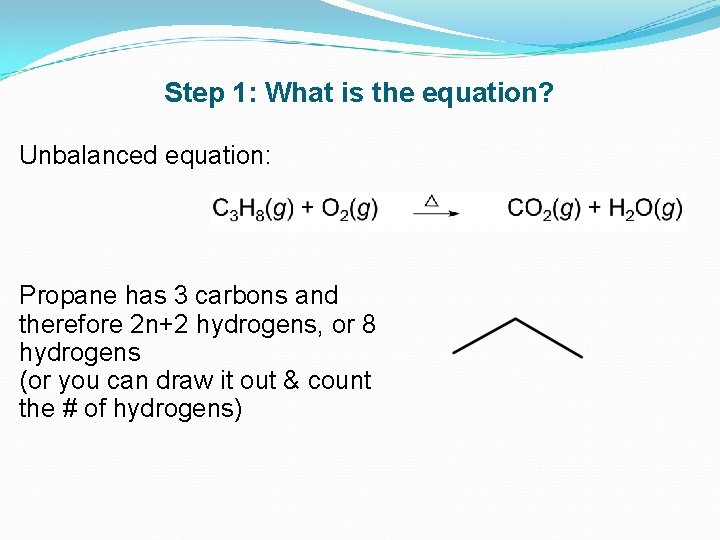
Propane chemical formula is C3H8. With unburned components in the exhaust gas such as C H 2 CO the combustion process is uncompleted and not stoichiometric. For example the equation for converting from methanes emissions factors to propanes emissions.
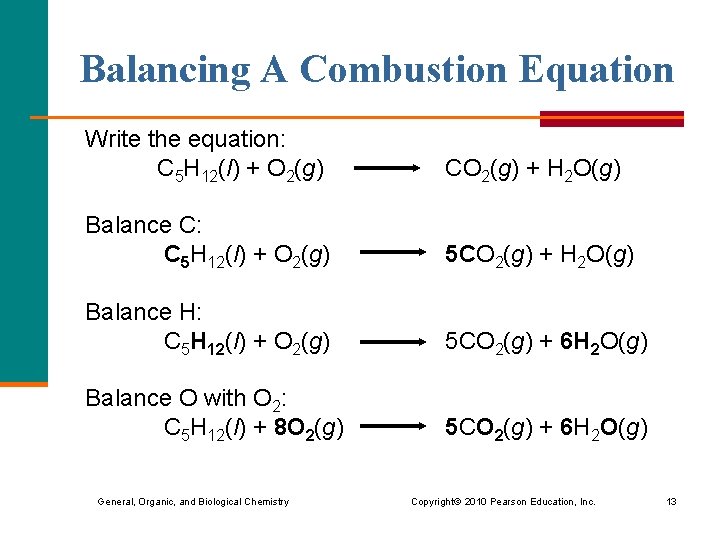
Combustion equation for Propane - C3H8 5O2 - 3CO2 4H2O for complete combustion. 4 The reaction can be summarized in the following chemical and word equations. Oxygen is essential for combustion and is used with ethyne acetylene in high-temperature oxyacetylene welding and cutting torches.
Propane ˈ p r oʊ p eɪ n is a three-carbon alkane with the molecular formula C 3 H 8It is a gas at standard temperature and pressure but compressible to a transportable liquidA by-product of natural gas processing and petroleum refining it is commonly used as a fuel in domestic and industrial applications and in low-emissions public transportation. Butane and Isobutane both have the same chemical formula C4H10 as isobutane is an isomer of butane. Heavier hydrocarbons pentanes plus are liquids or waxy solids.
The Henrys Law constant for propane is estimated as 707X10-1 atm-cu mmole SRC derived from its vapor pressure 7150 mm Hg 1 and water solubility 624 mgL 2. Solving the equation we get x 1016 k J m o l. AnswerC3H8g 5O2g 3CO2g 4H2OgExplanation.
The general equation for flame burning velocity S u by Andrews and Bradley 29 is given in equation 1 whereby the flame speed S f is related to burning velocity during the pre-pressure period and can be found experimentally. Armed only with the chemical formula for propane I walk through how to balance the equation of propane combustion and then calculate the energy released per. Youre dealing with the combustion of propane.