Sensational Penny Battery Chemical Equation

Zn 2 MnO 2 H 2 O ZnO 2 MnOOH.
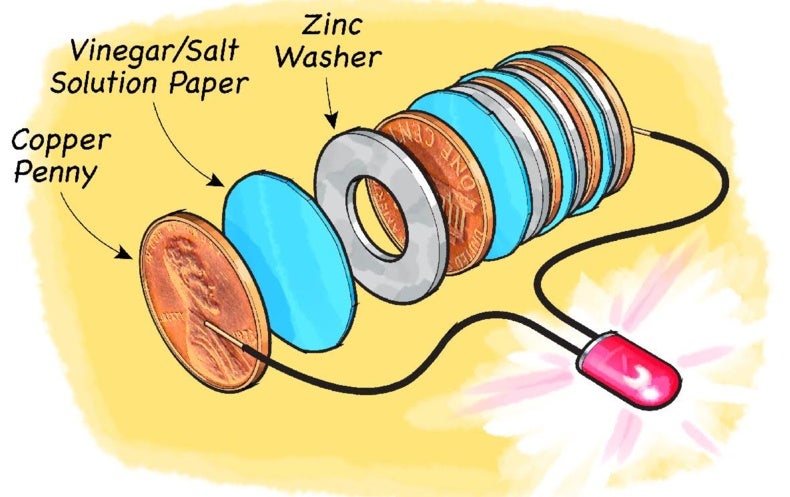
Penny battery chemical equation. Cut 3 circles of paper towel about the same diameter or slightly larger than the coins. The coin battery is designed as a stand-alone project but if the Light Bulb experiment is performed at the same time that can help connect whats happening on the voltmeters display to lighting the bulb in a flashlight. Penny 2 nickels and dime.
How it first came to be. Dip one penny halfway into the liquid. You can find wet cell batteries in cars.
Make a stack alternating coins and NaCl-soaked paper towel pieces. Through Coin Cell Testing An Undergraduate Honors Thesis. Common battery chemistries include.
Stir until the salt dissolves. Since the chemical reaction in the battery cells the capacity of a battery usually depends on the discharge conditions such discharging current rate temperature and other factors. Pennies get dull over time because the copper in the pennies slowly reacts with air to form copper oxide.
Modern batteries use a variety of chemicals to power their reactions. Obtain four coins from the front bench. Cut 3 circles of paper towel about the same diameter or slightly larger than the coins.
A small glass or bowl. The energy for the battery does not come from the lemon but rather the chemical change in zinc or other metal. Put the salt and vinegar in the bowl.