Smart Ammonia Hydrogen Chloride Balanced Equation

Balance oxygen last since it is present in more.
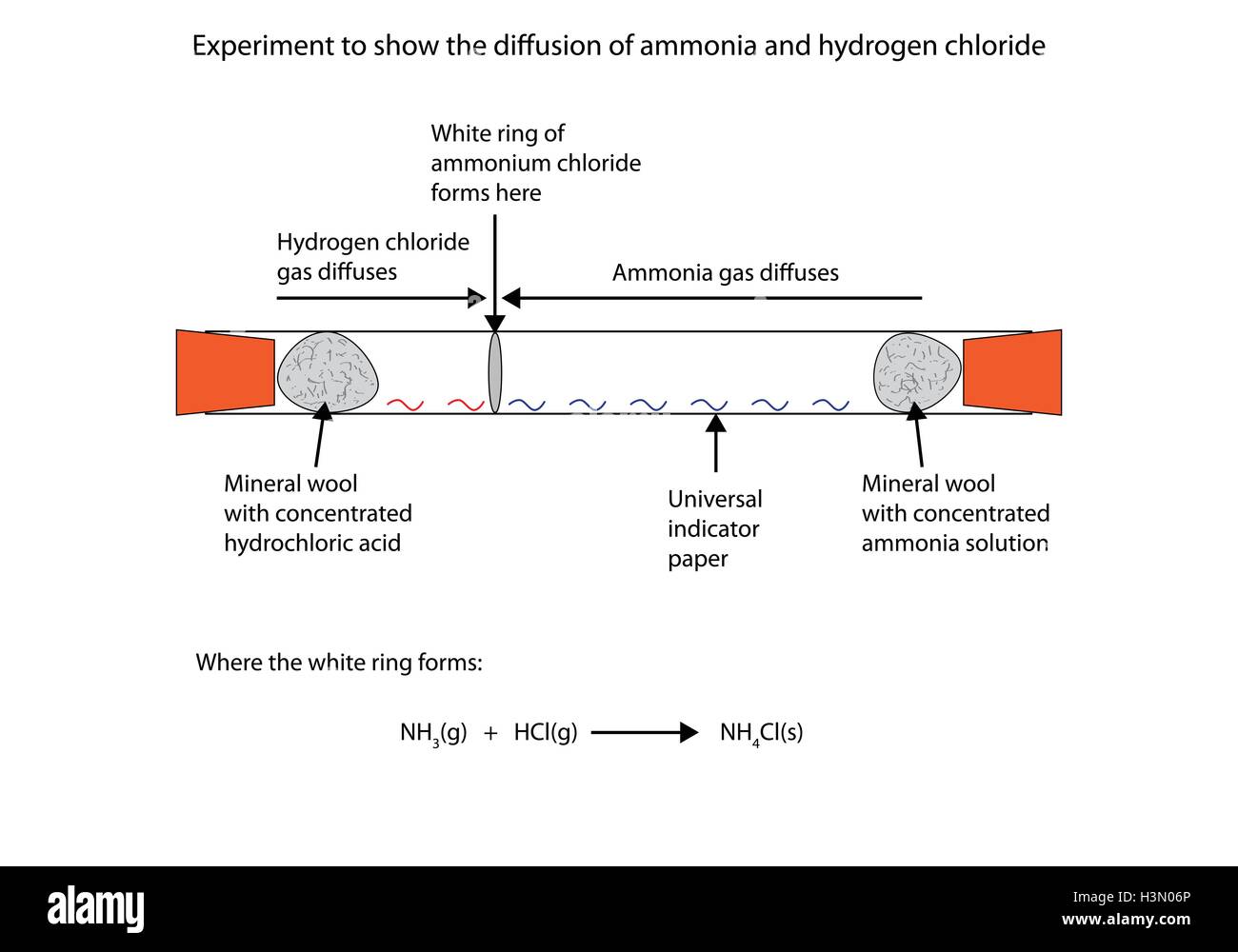
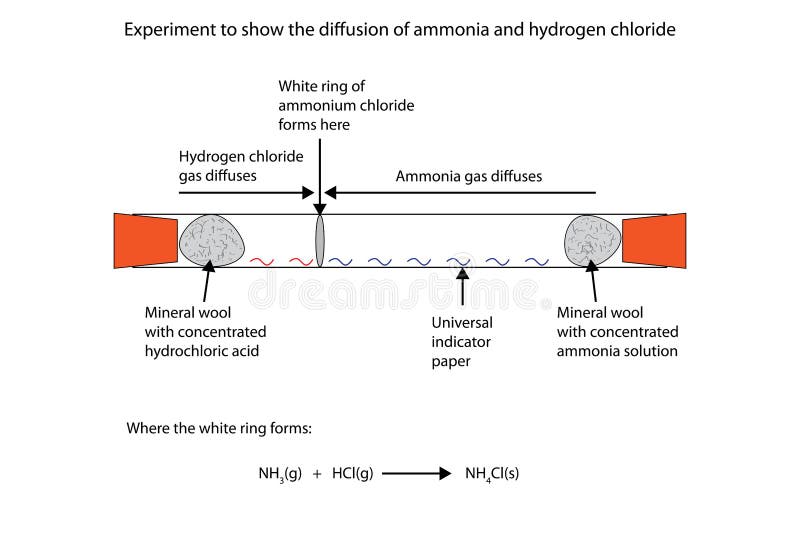
Ammonia hydrogen chloride balanced equation. The formula for ammonia is NH 3 so we have to take nitrogen and hydrogen in a ratio in which this formula is satisfied while the number of atoms of each element remain the same on both sides. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. It also shows that ammonia and hydrogen chloride.
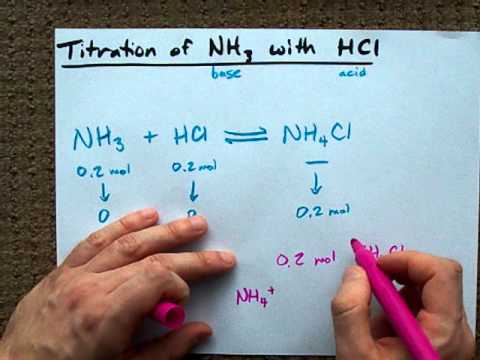
NH3g HCl g NH4Cls. B Sodium hydroxide solution is treated with acetic acid to form sodium acetate and water. Then hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which is a solid white smog.
Ammonia gas reacts with hydrogen chloride. D Potassium metal react with water to. Maharashtra State Board SSC English Medium 10th Standard Board Exam.
In this demo that means that the ammonia makes it to the hydrogen chloride flask before the hydrogen chloride has a chance to make it to the ammonia flask and we observe the reaction taking place above the HCl. Write a balanced chemical equation for the reaction and calculate the number of grams of excess reactant when 30 g of NH 3 reacts with 50 g of HCl. The balanced equation for the reaction between nitrogen and hydrogen is.
115 PSV 03 16. Here only chlorine atoms should be considered. Write an equation for this reaction.
According to the a balanced reaction equation 1 mole of ammonia gas reacts with exactly 1 mole of hydrogen chloride gas to produce the solid ammonium chloride. Ammonia gas reacts with hydrogen chloride. B Hydrogen sulphide gas burns in air to give water and sulphur dioxide c Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate.