Outrageous Hand Warmers Chemical Equation

Lets find out whats going on inside the pouch.
Hand warmers chemical equation. These handtoe warmers heat up in less than 30 minutes and provide up to 8 hours of continuous natural heat for your pockets gloves or boots. There are two types of chemical hand warmers that Im going to look at here. Hand warmers you buy usually have a porous pouch containing a mix of iron powder activated charcoal salt and vermiculite.
You shake it up and it starts to warm and later on as it wears down more shaking will squeeze out more heat. Hotsnapz Hand Warmers Reusable Round Pocket Warmers. Each pouch typically contains iron powder salt water an.
The packets of chemicals produce heat from oxidizing iron into iron oxide Fe 2 O 3 or rust. The heat given off by an air activated Hand warmer is caused by a chemical reaction. Oxidation-based hand warmer packs usually contain iron powder water salt activated carbon and.
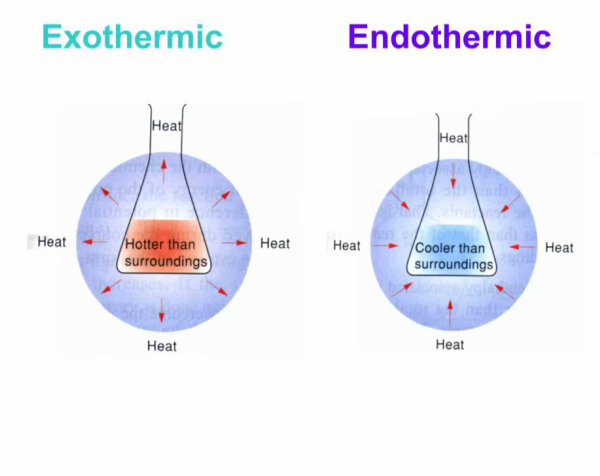
Other types of warmers are available to provide soothing heat for muscular or joint aches. SODIUM CHLORIDE Chemical equation Iron Powder 4Fes302g- 2Fe203s Like in your kitchen salt amplifies things. Disposable hand warmers turn up the heat in your mittens by means of an exothermic reaction that in essence just creates rust.
Figure 515 Chemical hand warmers produce heat that warms your hand on a cold day. Hand warmers work because of a rusting process. Popular hand warmers rely on heat-releasing chemical reactions also known as exothermic reactions.
The first are the more common disposable variety. There are several types of hand warmers commercially available that take advantage of different chemical reactions to provide a gentle heat for an extended period of time. They are commonly used in outdoor activities.