Outstanding Observation Of Burning Match Or Splint

CO2 and H2O are made by carbon and hydrogen in the burning wood.
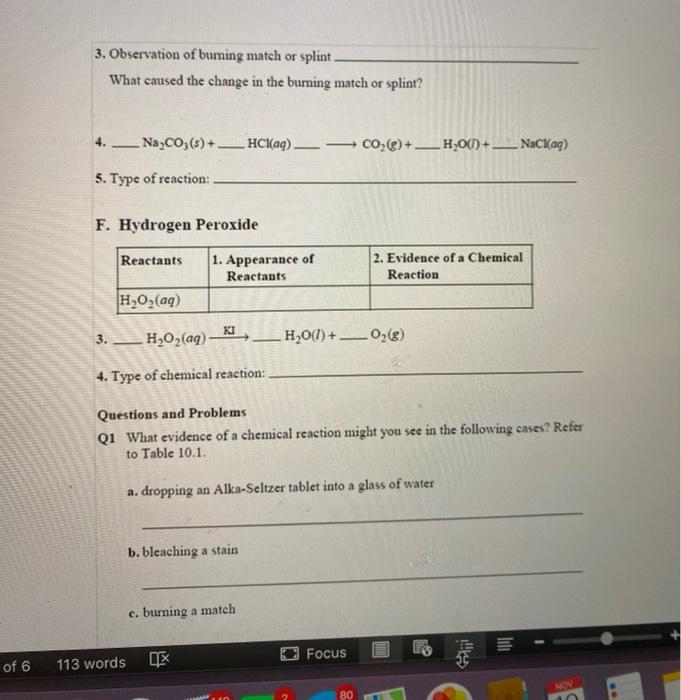
Observation of burning match or splint. The CO2 formed during the reaction displacing O2. Evidence Of A Chemical Reaction Formation Of Bubbles H2O2aq KI 02 3. Hydrogen Peroxide Reactants 1.
It will extinguish a flame more dense than air an does not support combustion burning splint goes into CO2 it goes out A glowing splint needs oxygen to basically keep glowing. Observation of burning match or splint. A Burning Splint Oxygen comprises about 20 of the air we breathe and even the air we dont breathe.
NaC0 - HC CO H00 NaCl 5. Na2CO36 __HClaq 5. Why did the flame of the burning match or splint go out.
However many of these combustion reactions occur much more readily in an atmosphere of pure oxygen. CO2 H20m NaClaq 4. It Immediately Went Out.
Oxygen gas rekindles the glowing splint as it bursts into flames. What caused the change in the burning match or splint. Why are burning candles and rusting nails examples of chemical change.
A glowing splint no flames burning is used to test for oxygen. NaC0 - HC CO H00 NaCl 5. Rust is oxidation.