Perfect Endothermic Reaction Equation Examples

NH4Cl s H2O l NH4Cl aq Heat.
Endothermic reaction equation examples. Since these are endothermic processes they will. There are many possible ingredients in an instant cold pack but they often contain solid ammonium nitrate and water. Mechanism of endothermic reactions In chemistry laboratories it is common to deal with endothermic reactions such as hydrolysis of water or the production of molecular chlorine from hydrochloric acid.
In our daily life it is also possible to find examples of such reactions. This is an example of an endothermic reaction. Heat location is the quickest way to find if a reaction is endothermic or exothermic.
Fusion of snow on a warm windshield especially for heavy snow. The equation for this reaction is. An instant cold pack is the perfect example of an endothermic reaction.
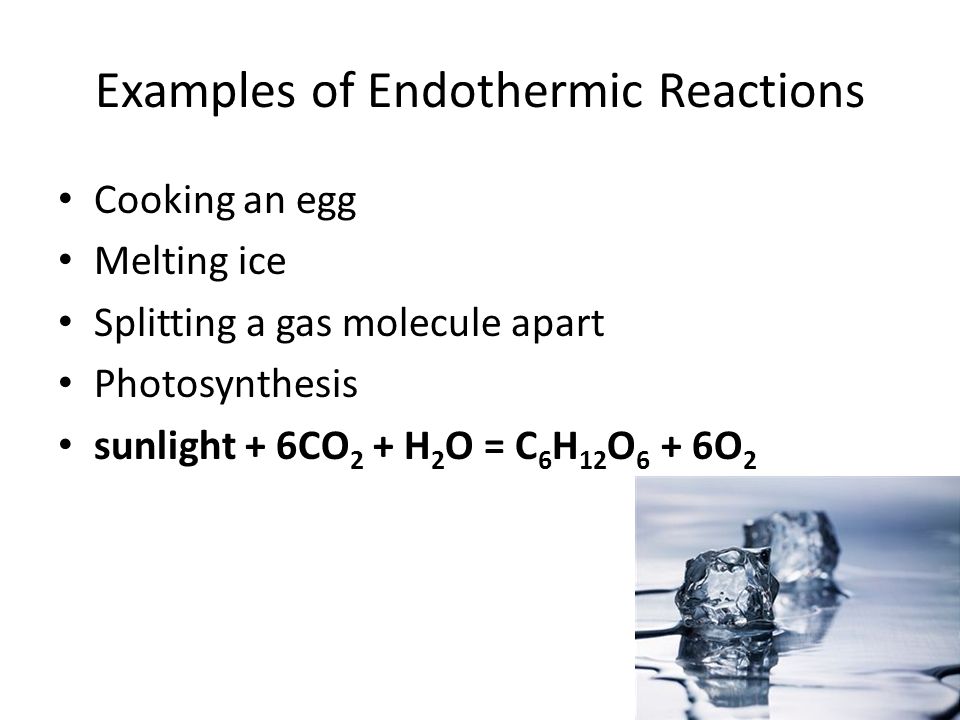
One of the most important series of endothermic reactions is photosynthesis. Sunlight 6CO 2 g H 2 O l C 6 H 12 O 6 aq 6O 2 g. A few examples of the endothermic process are photosynthesis evaporating liquids melting ice dry ice alkanes cracking thermal decomposition ammonium chloride in water and much more.
This is actually one of the key characteristics of an endothermic reaction. A constant input of energy often in the form of heat is needed to keep an endothermic reaction going. Examples of endothermic reactions include photosynthesis which uses sunlight and melting ice cubes which uses heat.
Photosynthesis is an example of an endothermic chemical reaction. Chemical reactions are all about the energy. Since this physical change cannot occur without the heat input from surroundings this phenomenon is classified as an endothermic process.