Heartwarming Naoh Hcl Balanced Equation

This means that we will split them apart in the net ionic equation.
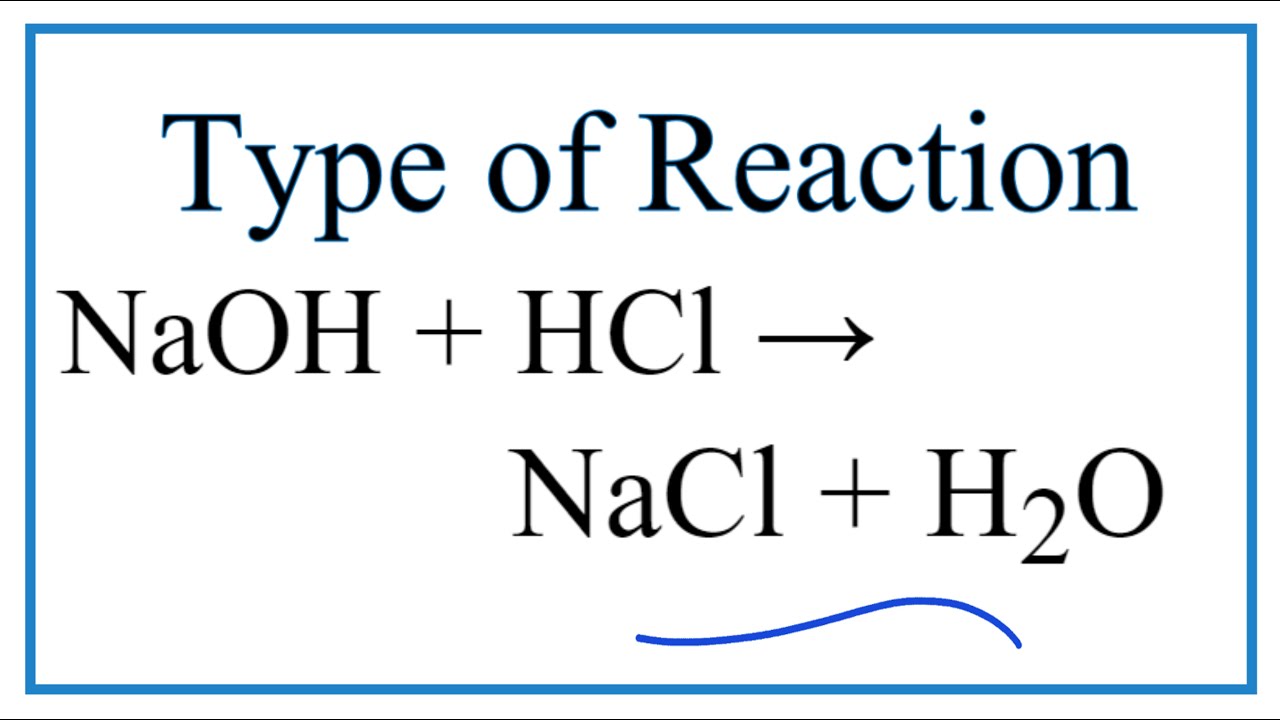
Naoh hcl balanced equation. K 4 FeCN 6 H 2 SO 4 H 2 O K 2 SO 4 FeSO 4 NH 4. What is the product of NaOH HCl. In this equation they react to form the products NaCL sodium chloride or salt and H2O water.
H OH - H 2 O Positive sodium ions from NaOH and negative chloride ions from HCL combine to form the salt sodium chloride NaCl commonly called table salt. Energy cannot be created or destroyed but it can be exchanged. 2 clean small test tubes NHACI Litmus paper Test tube rack 30 MHCI CaCO3 30 M NaOH 1.
To balance a chemical equation enter an equation of a chemical reaction and press the Balance button. Hence the balanced chemical equation is shown below. HCl aq NaOH aq NaCl aq H2O l heat Calculate the number of moles of base you add to determine the molar heat of neutralization expressed using the equation ΔH Q n where n is the number of moles.
What happens when HCl is added to NaCl. The heat of neutralization for the reaction of HCl with NaOH is. The limiting reagent row will be highlighted in pink.
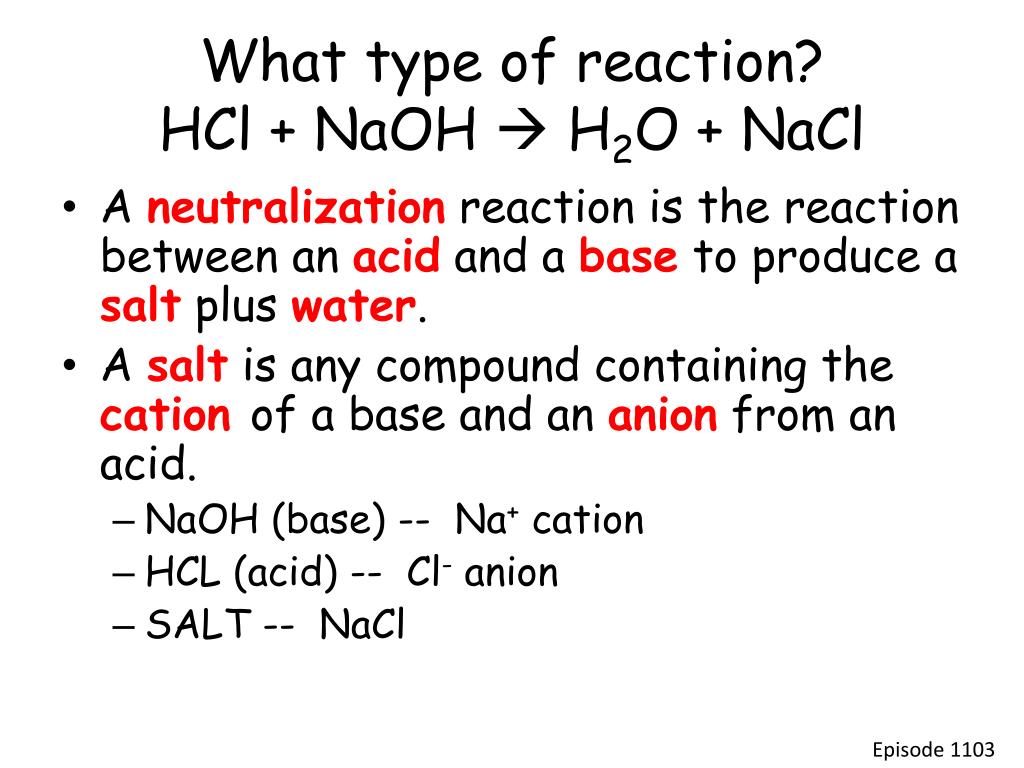
Then we can continue and learn. NaOHaqHClaq NaClaqH2Ol N a O H a q H C l a q N a C l a q H 2 O l NaOH HCland NaCl N a O H H C l a n d N a C l are. The balanced chemical equation representing the neutralization of hydrochloric acid with sodium hydroxide is.
Fe Cl 2 FeCl 3. But before that I think it is a good idea to look into your solubility rules. KMnO 4 HCl KCl MnCl 2 H 2 O Cl 2.