Amazing Exothermic Equations Examples
Reaction between two gases.
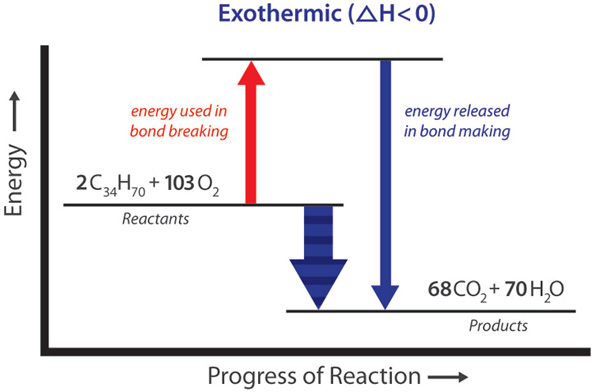
Exothermic equations examples. When a chemical reaction occurs energy is transferred to or from the surroundings. Firing a firecracker the bursting of a firecracker gives out a loud noise in addition to light and heat being one of the best examples of an exothermic reaction. That is the energy needed to initiate the reaction is less than the energy released.
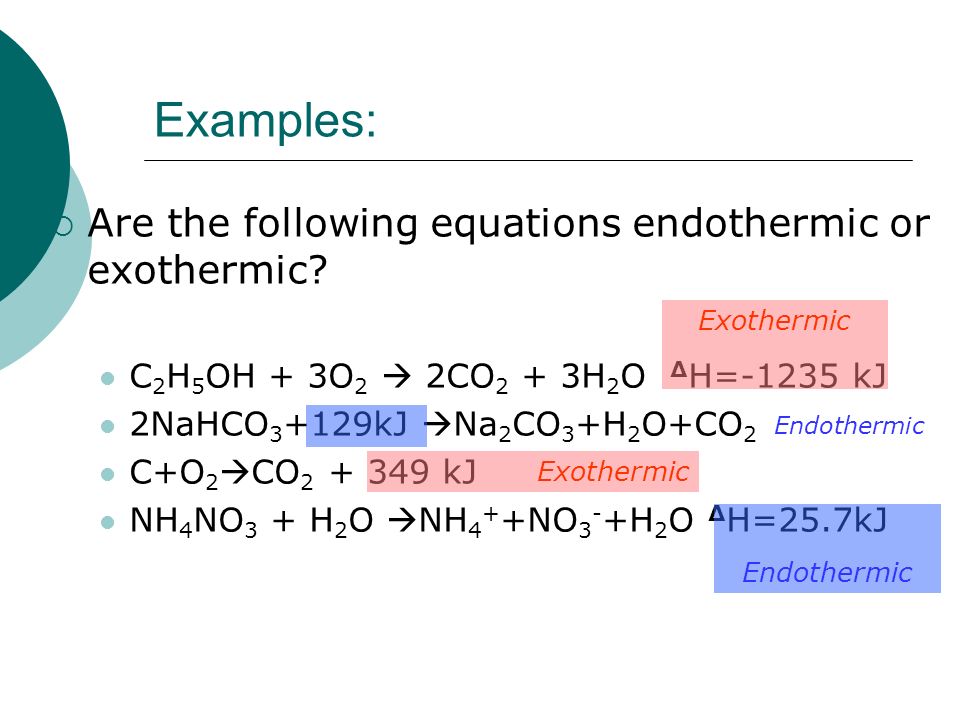
Q8 Give examples of exothermic reactions ie. An exothermic reaction is defined as a reaction that releases heat and has a net negative standard enthalpy change. Why is the formation of co2 exothermic.
Reaction between a solid and a liquid. B Is oxidation an exothermic or an endothermic reaction. Heat energy is absorbed from the pan to cook the egg.
C Explain by giving an example low oxidation and reduction proceed side by. The fire example above is intuitive as energy is clearly being released into the environment. Ultimately the yeast which are facultative fungal organisms provide enzymes that break down sugar molecules while releasing Ethanol and Carbon Dioxide as by-products through the exothermic reaction.
The balanced chemical equations are shown along with the examples 156. Exothermic reaction - Wikipedia Examples are numerous. 2H 2 g O 2 g 2H 2 O g and the respective enthalpy change of this reaction is.
Heat evolved in each of the following. 6CO2 6 H2O heat --- C6H12O6 6O2. An Exothermic reaction is a chemical reaction that involves the release of energy in the form of heat or light.