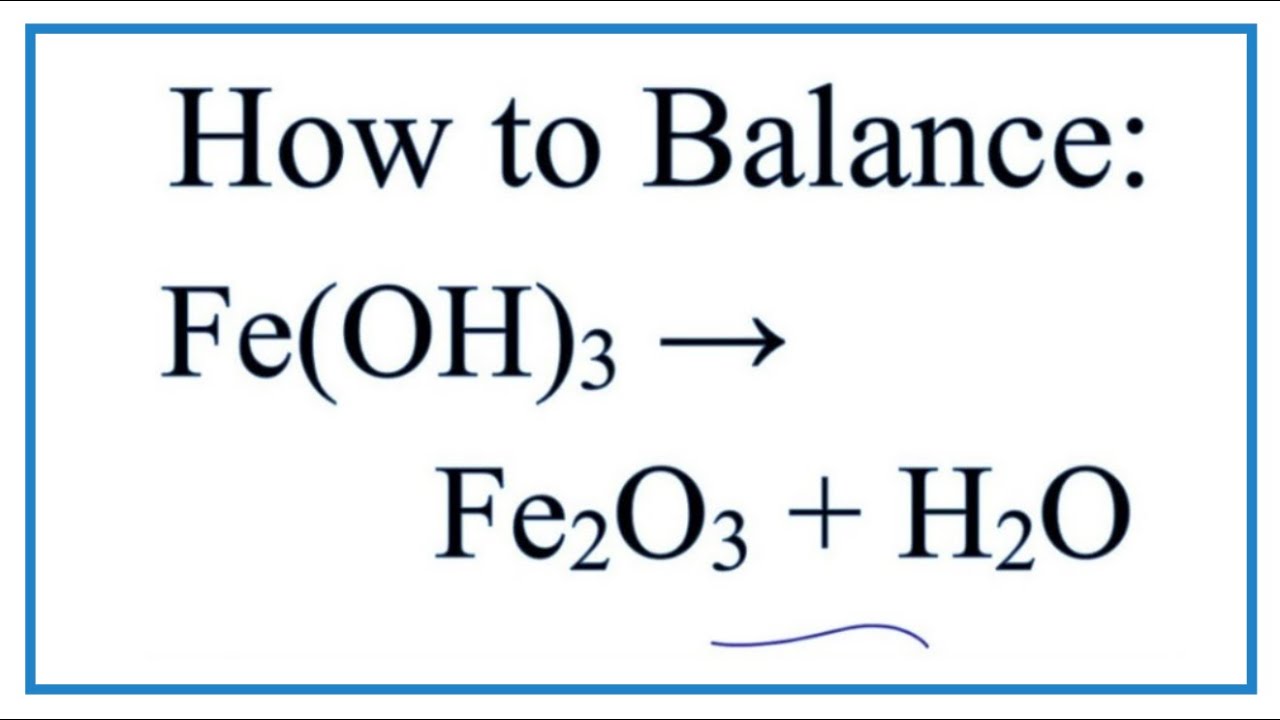
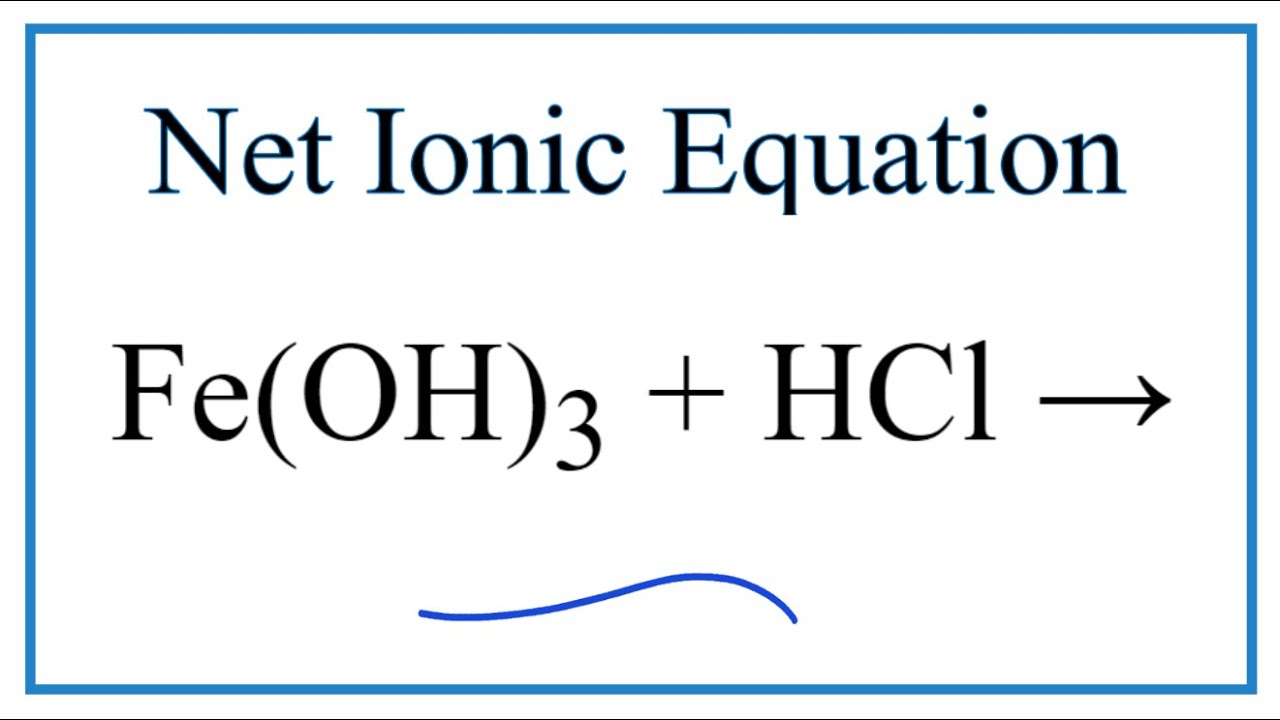First Class Is Feoh A Strong Base
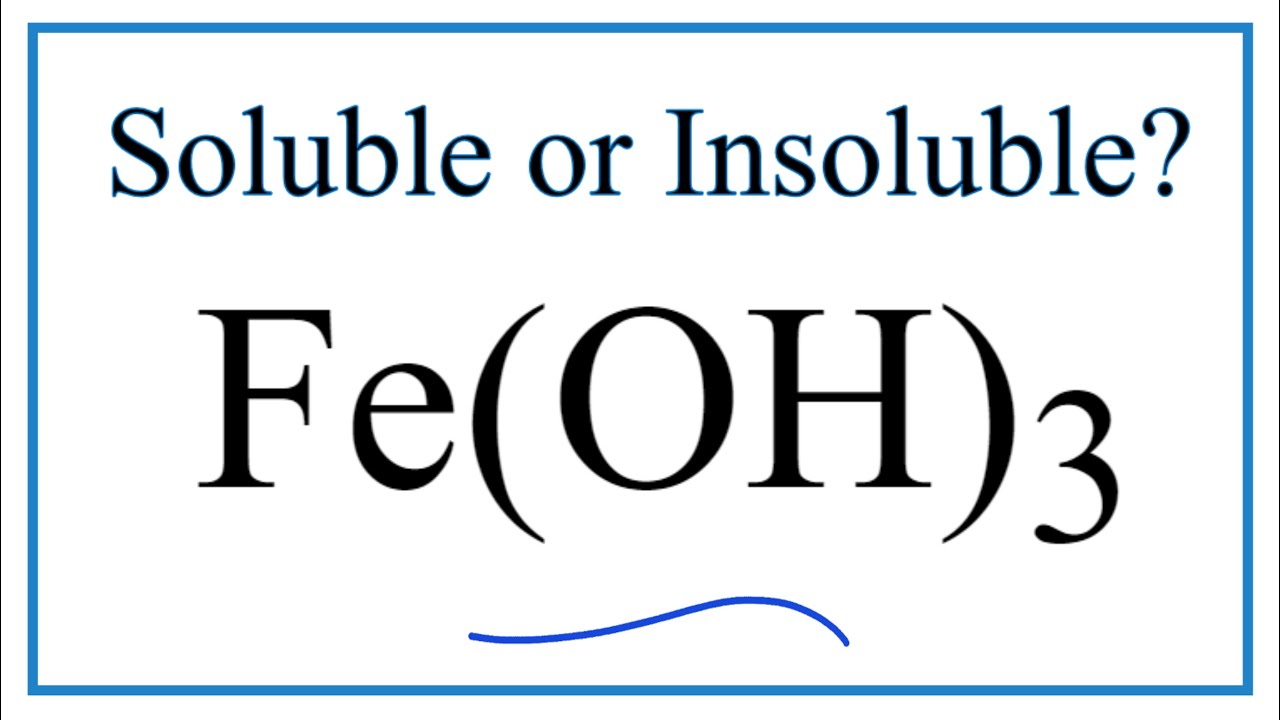
Subsequently question is is iron hydroxide soluble in water.
Is feoh a strong base. In a reaction between an acid and a base the products are water and a usually dissolved salt. However even though iron III hydroxide isnt very soluble a tiny portion does dissolve. Yes the reaction occurs because FeOHis a weak base.
Because MgOH 2 is listed in Table 122 Strong Acids and Bases it is a strong base. And a transition metal compound and a hydroxide and. Fe OH3 can be considered as base not an alkali.
Compounds do not class themselves as either one thing or another. Identify each acid or base as strong or weak. For a list of common weak acids and bases see Table 8-2 in Oxtoby8-2 in Oxtoby.
Use of the information documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice and subject to other binding limitations provided for under applicable law the information documents and data made available on the ECHA website may be reproduced distributed andor used totally or in part for non-commercial purposes provided that ECHA is. FeOH2 IronII hydroxide is Insoluble in water What is Soluble and Insoluble. In chemistry neutralization or neutralisation see spelling differences is a chemical reaction in which an acid and a base react quantitatively with each other.
If you want to quickly find the word you want to search use Ctrl F then type the word you want to search. Iron II hydroxide FeOH2 is very insoluble in water. MgOH 2 C 5 H 5 N.
LIST ACID NH4ClO4 NH4Cl HBrO WEAK H2PO4-H3PO3 WEAK HNO3 STRONG HCl STRONG H2S WEAK H2SO4 STRONG H3PO4 WEAK H2CO3 WEAK HBr STRONG HI STRONG HClO4 STRONG HClO3. Learn bases strong weak strong vs with free interactive flashcards. The salt consists of ions that would be the conjugate base of.