Outrageous Hydrogen Chloride And Ammonia Balanced Equation

Balance oxygen last since it is present in more.
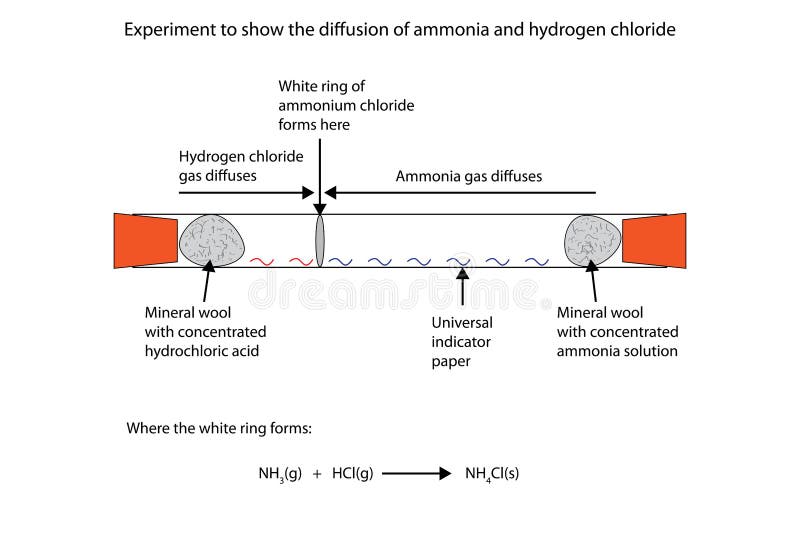
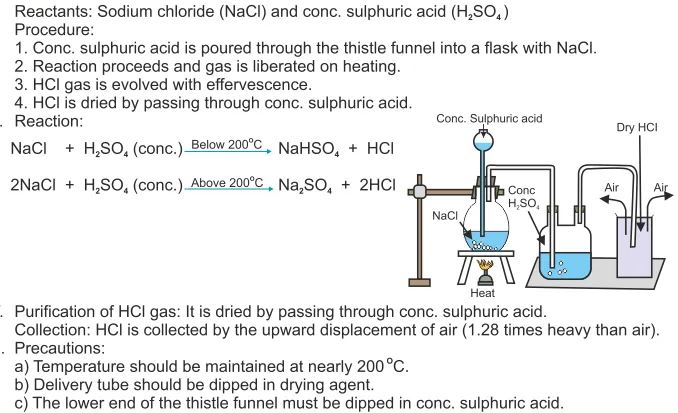
Hydrogen chloride and ammonia balanced equation. The reaction equation between ammonia NH3 and hydrochloric acid HCl is written as follows. Write a balanced chemical equation for the reaction and calculate the number of grams of excess reactant when 30 g of NH 3 reacts with 50 g of HCl. First ammonia reacts with chlorine and produce nitrogen gas and hydrogen chloride vapor.
Sulfurous acid hydrogen ammonium salt and products h2o water nh4cl ammonium chloride so2 sulfur dioxide. Or if any of the following reactant substances NiNH36Cl2 disappearing. Write a balanced equation for the reaction of gaseous ammonia with hydrogen chloride asked Jun 26 2017 in Chemistry by Clotto A NH3g HClg - NH2Clg H2g.
H2 Cl2 2HCl. Translate the following statements into chemical equations and then balance them. One may also ask what is the chemical reaction for ammonia.
Then hydrogen chloride reacts with basic ammonia gas to produce ammonium chloride which is. The pressure of a container is a result of the collisions of gas molecules with the walls of the container. Write a balanced equation for the reaction of gaseous ammonia with hydrogen chloride.
Asked Mar 5 2019 in Chemistry by LadyBugMichelle A. Condition No information found for this chemical equation Phenomenon. Ammonia is a weak base that reacts with hydrochloric acid forming a compound called ammonium chloride.
Here only chlorine atoms should be considered. The chemical equation for the decomposition of ammonia is. 2H2 O2 2H2O 3.