Spectacular Formula For Endothermic Reaction

It explains the flow of heat energy into and out of the system and surroundin.
Formula for endothermic reaction. Also know what is the equation for an endothermic reaction. This chemistry video tutorial focuses on endothermic and exothermic reactions. Diagrams showing the systems and surroundings for exothermic and endothermic reactions 2020 Lets Talk Science.
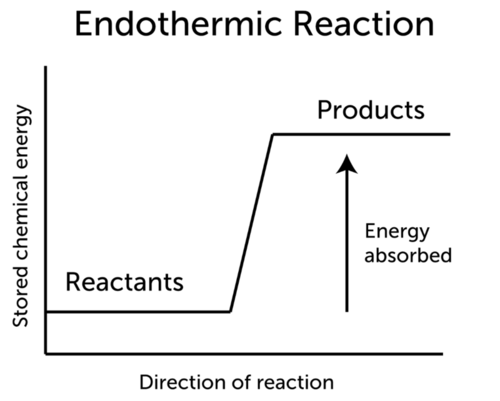
A constant input of energy often in the form of heat is needed to keep an endothermic reaction going. There are two sides to any chemical reaction. Look at the information given.
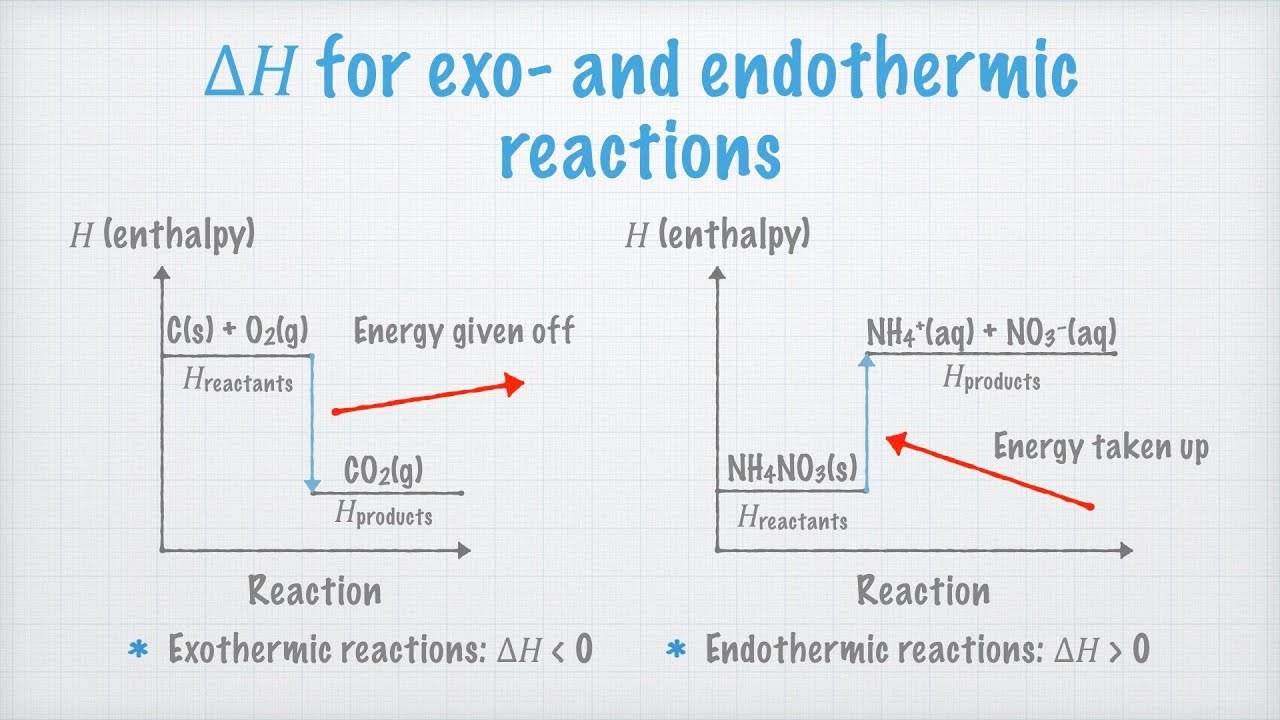
That explains why we feel hot when we stand beside a fire 1-3. An exothermic reaction causes the surroundings to heat up. In this reaction 43 kcal are needed to make the reaction occur.
The system reaction releases heat to the surroundings as the reactants transform into products. Plants absorb heat energy from sunlight to convert carbon dioxide and water into glucose and oxygen. It is possible to predict whether a reaction will be endothermic or exothermic by doing a little math.
N 2g O 2g 2NOg Cs 2Ss CS 2l. This video is very helpful for 10th board students. As a result the products are likely to be warmer than the reactants.
When ammonium chloride NH 4 Cl is dissolved in water an endothermic reaction takes place. The general equation for an exothermic reaction is. One of the most important series of endothermic reactions is photosynthesis.