Beautiful Work Chemical Equation For Fire Burning Wood

Printing photos for burning.
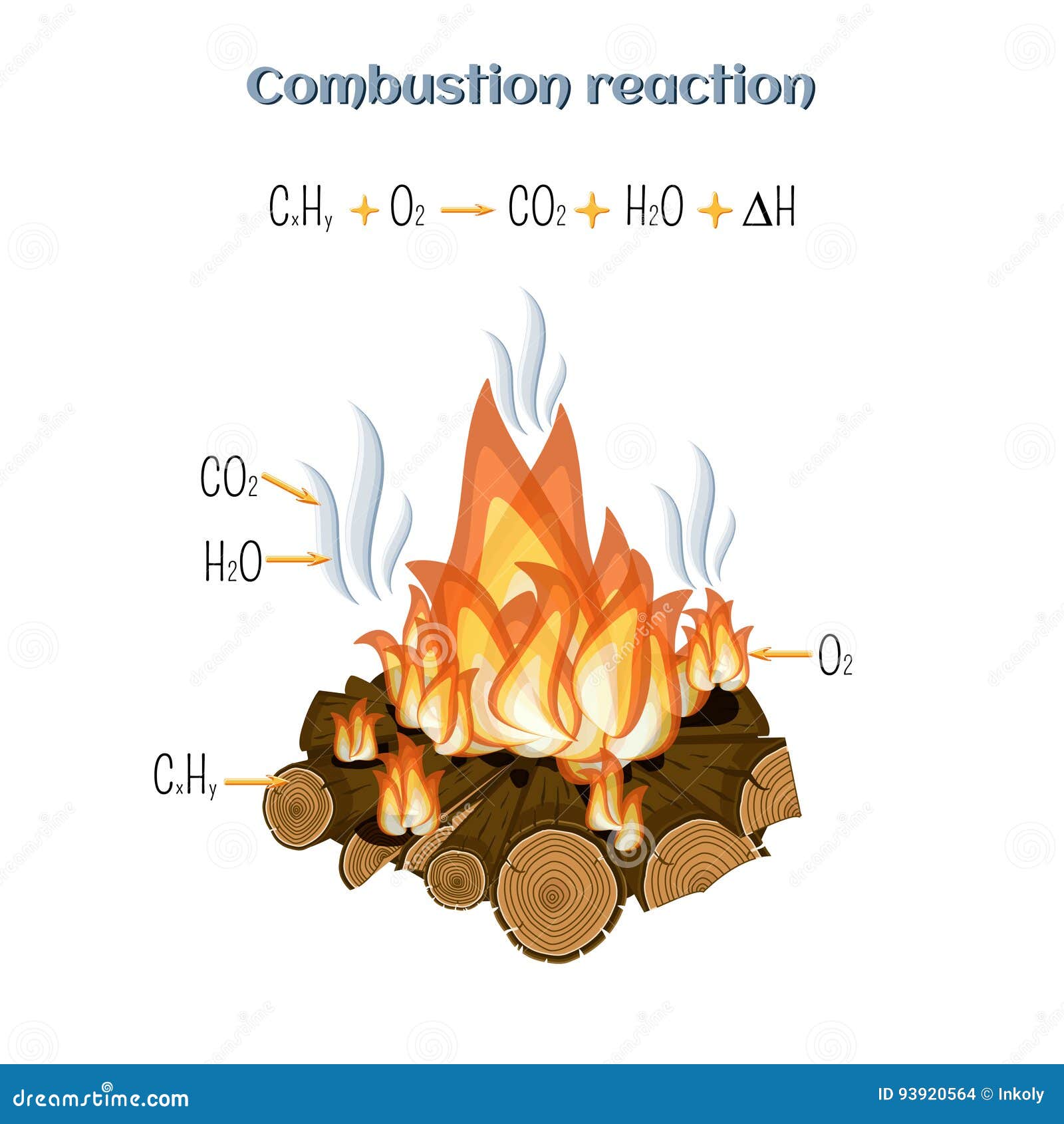
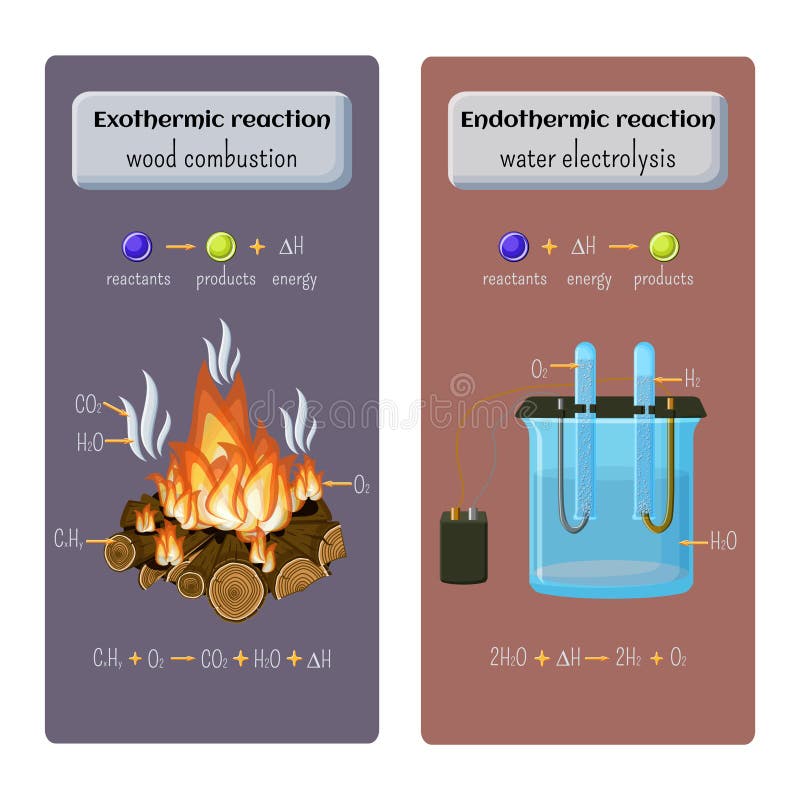
Chemical equation for fire burning wood. In this paper we will briefly review some of what is now known about the burning properties of wood and the resultant fire. The law of conservation of mass states that matter cannot be created or destroyed in a chemical reaction. But the equation for that is independent for this equat.
STEP 2 This is really an optional step but its helpful to lightly sketch your design with pencil. May 16 2011 at 1026 am Pity its unlikely that most inkjet carts would not survive the caustic. This accelerated oxidation is rusting.
Plume dynamics the amount and chemical composition of the emissions and the atmosphere into which the emissions are dispersed. So the mass of the product equals the mass of the reactant. Glucose is a sugar and cellulose formula is C 6 H 10 O 5 When burning wood reacts with Oxygen which is contained in air.
In the Americas in 1637 the people of Boston suffered from the scarcity of wood. Actually wood is composed mainly of Cellulose that is a polymer made up by repetition of Glucose residues. 4Fe 3O2 2Fe2O3.
Here is a look as some of the principal chemicals produced from wood smoke. In wood combustion this is the burning of the charcoalthe charcoal hot coalshot coals embers. The following chemical equation represents the reaction.
41 Wood History of Burning Wood. Beside above why is steel wool heavier when burned. Primary Combustion is the burning of solid material directly.