Divine Ch3oh Combustion Reaction

CH3 OH CH3OH and the insertion reaction.
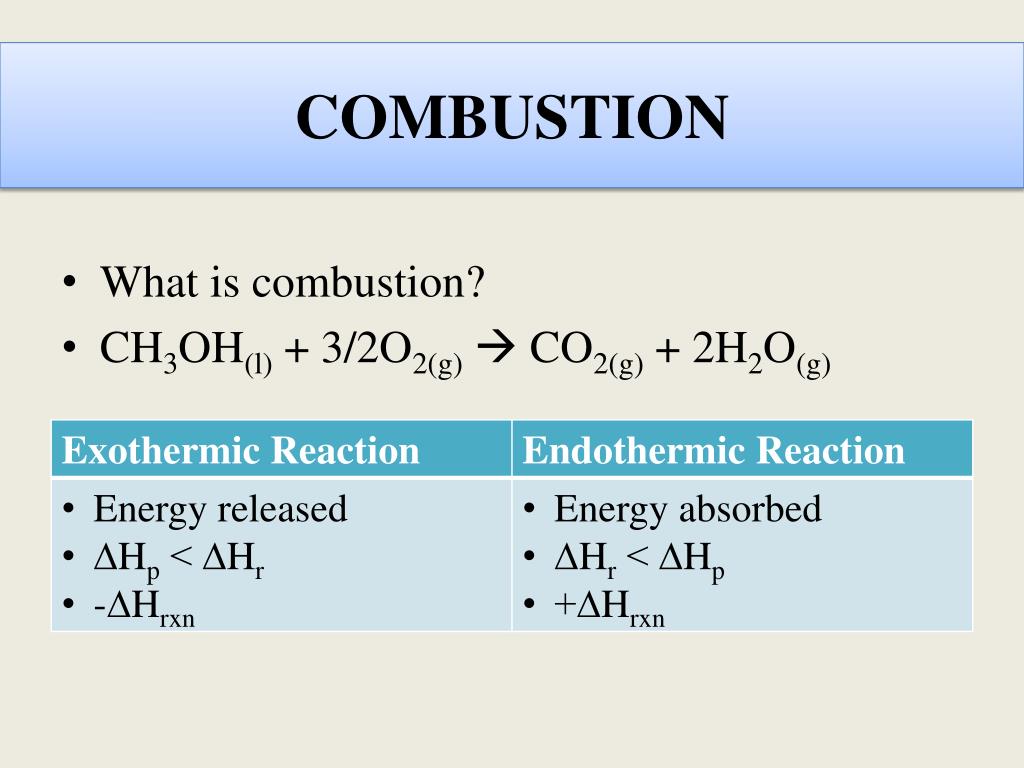
Ch3oh combustion reaction. The density of liquid methanol is 0792 gmL. Since the definitions of endothermic and exothermic reaction are pretty straightforward as far as energy movement is concerned it should be rather simple to deduce that when energy is released we are dealing with an exothermic reaction. Is 715 kJ mol1 and the heats of formation of carbon dioxide gas and water liquid are 3935 kJ mol1 and -2858 kJ mol1 respectively.
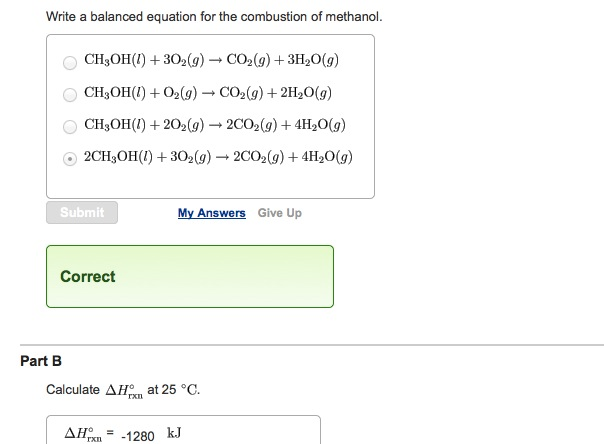
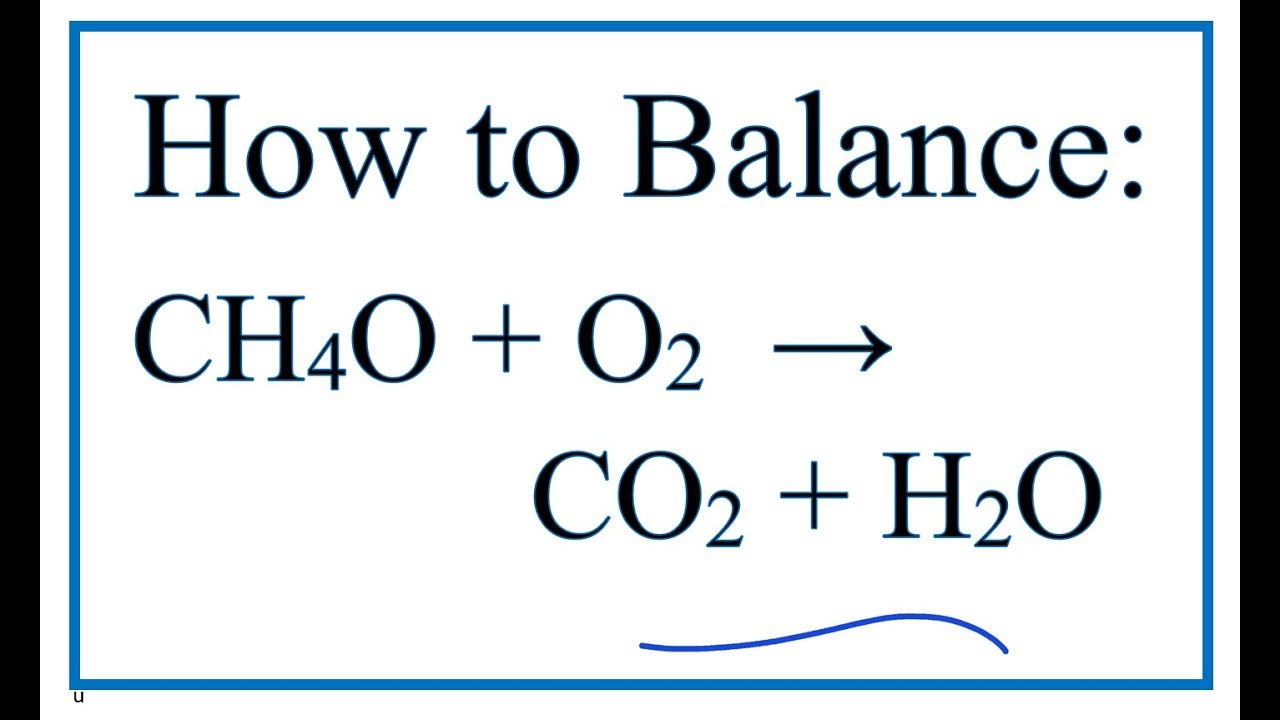
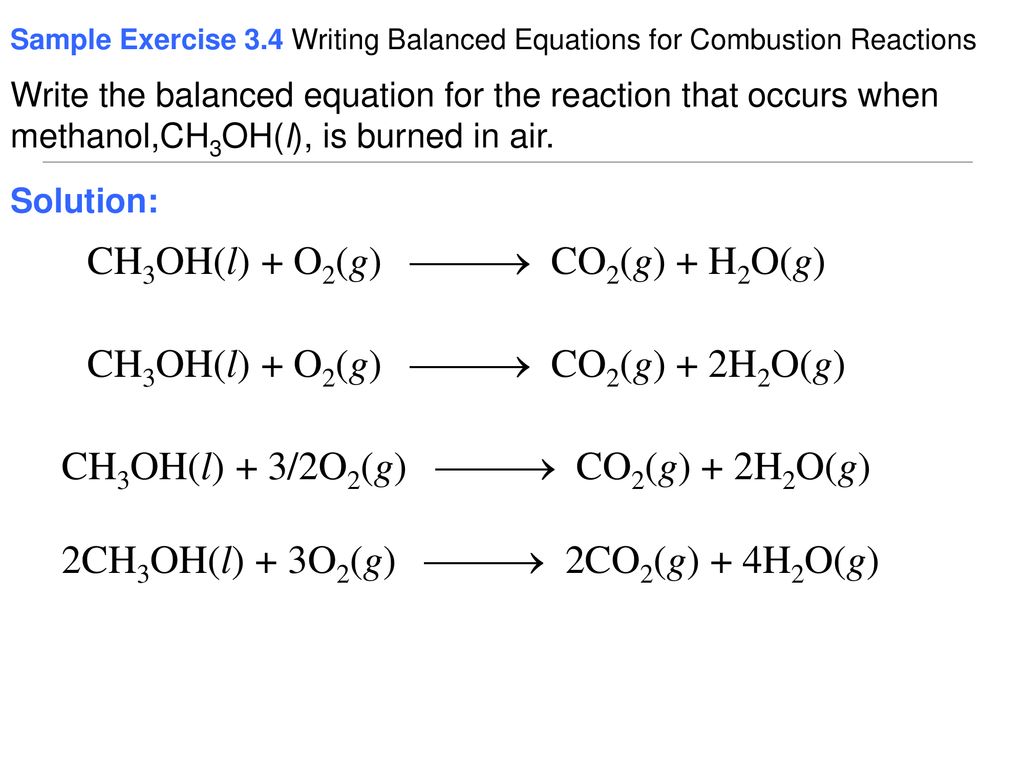
The unbalance equation will be. Here we focus on the ignition phase of the combustion and thus we adopt the auto-ignition delay time τ as. According to the following combustion reaction pure methanol liquid undergoes complete combustion with excess oxygen.
In this video we determine the type of chemical reaction for the equation CH3OH O2 CO2 H2O Combustion of MethanolSince we have a hydrocarbon plus Ox. Jayanks answer is perfectly good for introductory chemistry exams but the reality of the situation is more complicated. The combustion of methanol or any other hydrocarbon is not a single step reaction involving a single equation.
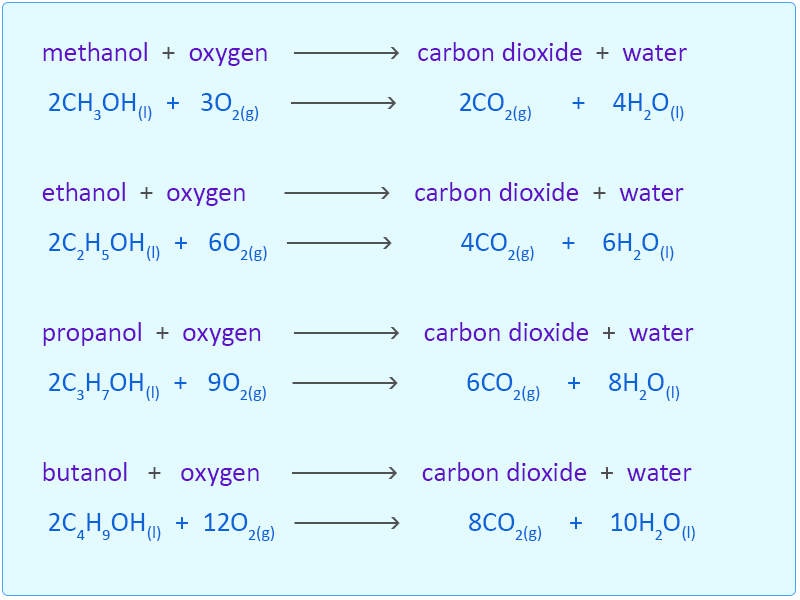
Sketch a potential energy diagram include title x and y-axis labels reactants and products endothermic or exothermic - 2 marks Question 4 4 points For the reaction. 2CH_3OHl 3O_2g rarr 2CO_2g 4H_2Ol If you multiply the coefficients the numbers in front times the subscripts for each element in each formula you will find that there are 2 atoms of carbon 8 atoms of hydrogen and 8. Top contributors to the provenance of Δ f H of CH3OH l The 20 contributors listed below account only for 783 of the provenance of Δ f H of CH3OH l.
Begingroup Regarding your second question - yes you have to infer from the questions phrasing the direction of energy movement. There found to be two pathways for the formation of methanol with about equal importance ie the recombination reaction. If 3992 liters L of pure carbon dioxide CO2 water removed was recovered at STP how many milliliters mL of methanol CH3OH must have reacted.
In the combustion of methanol in the presence of ample oxygen methanol will react with the oxygen to produce carbon dioxide and water. If 3928 liters L of pure carbon dioxide CO2 water removed was recovered at STP how many milliliters mL of methanol CH3OH must have reacted. 1½O2g CO2g 2H2O.